A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana
- PMID: 29891679
- PMCID: PMC6042063
- DOI: 10.1073/pnas.1803841115
A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana
Abstract
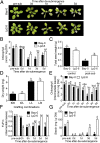
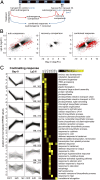
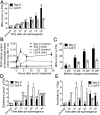
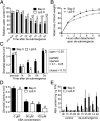
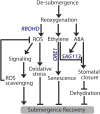
Abiotic stresses in plants are often transient, and the recovery phase following stress removal is critical. Flooding, a major abiotic stress that negatively impacts plant biodiversity and agriculture, is a sequential stress where tolerance is strongly dependent on viability underwater and during the postflooding period. Here we show that in Arabidopsis thaliana accessions (Bay-0 and Lp2-6), different rates of submergence recovery correlate with submergence tolerance and fecundity. A genome-wide assessment of ribosome-associated transcripts in Bay-0 and Lp2-6 revealed a signaling network regulating recovery processes. Differential recovery between the accessions was related to the activity of three genes: RESPIRATORY BURST OXIDASE HOMOLOG D, SENESCENCE-ASSOCIATED GENE113, and ORESARA1, which function in a regulatory network involving a reactive oxygen species (ROS) burst upon desubmergence and the hormones abscisic acid and ethylene. This regulatory module controls ROS homeostasis, stomatal aperture, and chlorophyll degradation during submergence recovery. This work uncovers a signaling network that regulates recovery processes following flooding to hasten the return to prestress homeostasis.
Keywords: dehydration; flooding; reactive oxygen species; recovery; ribosome footprinting.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Voesenek LACJ, Bailey-Serres J. Flood adaptive traits and processes: An overview. New Phytol. 2015;206:57–73. - PubMed
-
- Hirabayashi Y, et al. Global flood risk under climate change. Nat Clim Chang. 2013;3:816–821.
-
- Jackson MB. Ethylene and responses of plants to soil waterlogging and submergence. Annu Rev Plant Physiol. 1985;36:145–174.
-
- Armstrong W. Aeration in higher plants. Adv Bot Res. 1980;7:225–332.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

