Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters
- PMID: 29891683
- PMCID: PMC6042134
- DOI: 10.1073/pnas.1805358115
Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters
Abstract
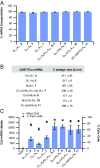
We report a strategy for generating a combinatorial library of oligonucleotide transporters with varied lipid domains and their use in the efficient transfection of lymphocytes with mRNA in vitro and in vivo. This library is based on amphiphilic charge-altering releasable transporters (CARTs) that contain a lipophilic block functionalized with various side-chain lipids and a polycationic α-amino ester mRNA-binding block that undergoes rearrangement to neutral small molecules, resulting in mRNA release. We show that certain binary mixtures of these lipid-varied CARTs provide up to a ninefold enhancement in mRNA translation in lymphocytes in vitro relative to either a single-lipid CART component alone or the commercial reagent Lipofectamine 2000, corresponding to a striking increase in percent transfection from 9-12% to 80%. Informed by the results with binary mixtures, we further show that CARTs consisting of optimized ratios of the two lead lipids incorporated into a single hybrid-lipid transporter molecule maintain the same delivery efficacy as the noncovalent mixture of two CARTs. The lead lipid CART mixtures and hybrid-lipid CARTs show enhanced lymphocyte transfection in primary T cells and in vivo in mice. This combinatorial approach for rapidly screening mRNA delivery vectors has provided lipid-varied CART mixtures and hybrid-lipid CARTs that exhibit significant improvement in mRNA delivery to lymphocytes, a finding of potentially broad value in research and clinical applications.
Keywords: combinatorial; gene therapy; immunotherapy; nanoparticle; polyplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Fingolimod-Conjugated Charge-Altering Releasable Transporters Efficiently and Specifically Deliver mRNA to Lymphocytes In Vivo and In Vitro.Biomacromolecules. 2022 Jul 11;23(7):2976-2988. doi: 10.1021/acs.biomac.2c00469. Epub 2022 Jun 24. Biomacromolecules. 2022. PMID: 35748182 Free PMC article.
-
Functional DNA Delivery Enabled by Lipid-Modified Charge-Altering Releasable Transporters (CARTs).Biomacromolecules. 2018 Jul 9;19(7):2812-2824. doi: 10.1021/acs.biomac.8b00401. Epub 2018 May 11. Biomacromolecules. 2018. PMID: 29727572 Free PMC article.
-
Isoprenoid CARTs: In Vitro and In Vivo mRNA Delivery by Charge-Altering Releasable Transporters Functionalized with Archaea-inspired Branched Lipids.Biomacromolecules. 2024 Jul 8;25(7):4305-4316. doi: 10.1021/acs.biomac.4c00373. Epub 2024 May 30. Biomacromolecules. 2024. PMID: 38814265 Free PMC article.
-
Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing.Acc Chem Res. 2021 Nov 2;54(21):4001-4011. doi: 10.1021/acs.accounts.1c00500. Epub 2021 Oct 20. Acc Chem Res. 2021. PMID: 34668716 Review.
-
Cationic lipid-DNA complexes for gene therapy: understanding the relationship between complex structure and gene delivery pathways at the molecular level.Curr Med Chem. 2004 Jan;11(2):133-49. doi: 10.2174/0929867043456160. Curr Med Chem. 2004. PMID: 14754413 Review.
Cited by
-
Advances and applications of RNA vaccines in tumor treatment.Mol Cancer. 2024 Oct 9;23(1):226. doi: 10.1186/s12943-024-02141-5. Mol Cancer. 2024. PMID: 39385255 Free PMC article. Review.
-
A Dual-Function Antibiotic-Transporter Conjugate Exhibits Superior Activity in Sterilizing MRSA Biofilms and Killing Persister Cells.J Am Chem Soc. 2018 Nov 28;140(47):16140-16151. doi: 10.1021/jacs.8b08711. Epub 2018 Nov 14. J Am Chem Soc. 2018. PMID: 30388366 Free PMC article.
-
Charge-Conversion Strategies for Nucleic Acid Delivery.Adv Funct Mater. 2021 Jun 9;31(24):2011103. doi: 10.1002/adfm.202011103. Epub 2021 Mar 31. Adv Funct Mater. 2021. PMID: 35832306 Free PMC article.
-
Targeted modulation of immune cells and tissues using engineered biomaterials.Nat Rev Bioeng. 2023 Feb;1(2):107-124. doi: 10.1038/s44222-022-00016-2. Epub 2023 Jan 30. Nat Rev Bioeng. 2023. PMID: 37772035 Free PMC article.
-
In situ T-cell transfection by anti-CD3-conjugated lipid nanoparticles leads to T-cell activation, migration, and phenotypic shift.Biomaterials. 2022 Feb;281:121339. doi: 10.1016/j.biomaterials.2021.121339. Epub 2021 Dec 29. Biomaterials. 2022. PMID: 35078042 Free PMC article.
References
-
- Friedmann T. A brief history of gene therapy. Nat Genet. 1992;2:93–98. - PubMed
-
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012–An update. J Gene Med. 2013;15:65–77. - PubMed
-
- Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. - PubMed
-
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics–Developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. - PubMed
-
- Meng Z, et al. A new developing class of gene delivery: Messenger RNA-based therapeutics. Biomater Sci. 2017;5:2381–2392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

