Primary productivity below the seafloor at deep-sea hot springs
- PMID: 29891698
- PMCID: PMC6042141
- DOI: 10.1073/pnas.1804351115
Primary productivity below the seafloor at deep-sea hot springs
Abstract
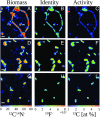
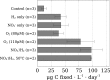
Below the seafloor at deep-sea hot springs, mixing of geothermal fluids with seawater supports a potentially vast microbial ecosystem. Although the identity of subseafloor microorganisms is largely known, their effect on deep-ocean biogeochemical cycles cannot be predicted without quantitative measurements of their metabolic rates and growth efficiency. Here, we report on incubations of subseafloor fluids under in situ conditions that quantitatively constrain subseafloor primary productivity, biomass standing stock, and turnover time. Single-cell-based activity measurements and 16S rRNA-gene analysis showed that Campylobacteria dominated carbon fixation and that oxygen concentration and temperature drove niche partitioning of closely related phylotypes. Our data reveal a very active subseafloor biosphere that fixes carbon at a rate of up to 321 μg C⋅L-1⋅d-1, turns over rapidly within tens of hours, rivals the productivity of chemosynthetic symbioses above the seafloor, and significantly influences deep-ocean biogeochemical cycling.
Keywords: Campylobacteria; NanoSIMS; chemosynthesis; deep-sea hydrothermal vents; ecophysiology.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Corliss JB, et al. Submarine thermal springs on the Galapagos rift. Science. 1979;203:1073–1083. - PubMed
-
- Karl DM, Wirsen CO, Jannasch HW. Deep-sea primary production at the Galapagos hydrothermal vents. Science. 1980;207:1345–1347.
-
- Wirsen CO, Jannasch HW, Molyneaux SJ. Chemosynthetic microbial activity at Mid-Atlantic ridge hydrothermal vent sites. J Geophys Res. 1993;98:9693–9703.
-
- Wirsen CO, Tuttle JH, Jannasch HW. Activities of sulfur-oxidizing bacteria at the 21 N East Pacific rise vent site. Mar Biol. 1986;92:449–456.
-
- Huber JA, et al. Microbial population structures in the deep marine biosphere. Science. 2007;318:97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

