Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy
- PMID: 29891713
- PMCID: PMC6042062
- DOI: 10.1073/pnas.1803907115
Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy
Abstract
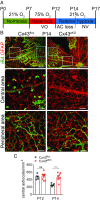
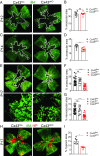
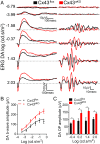
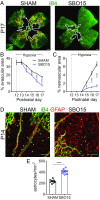
Degeneration of retinal astrocytes precedes hypoxia-driven pathologic neovascularization and vascular leakage in ischemic retinopathies. However, the molecular events that underlie astrocyte loss remain unclear. Astrocytes abundantly express connexin 43 (Cx43), a transmembrane protein that forms gap junction (GJ) channels and hemichannels. Cx channels can transfer toxic signals from dying cells to healthy neighbors under pathologic conditions. Here we show that Cx43 plays a critical role in astrocyte apoptosis and the resulting preretinal neovascularization in a mouse model of oxygen-induced retinopathy. Opening of Cx43 hemichannels was not observed following hypoxia. In contrast, GJ coupling between astrocytes increased, which could lead to amplification of injury. Accordingly, conditional deletion of Cx43 maintained a higher density of astrocytes in the hypoxic retina. We also identify a role for Cx43 phosphorylation in mediating these processes. Increased coupling in response to hypoxia is due to phosphorylation of Cx43 by casein kinase 1δ (CK1δ). Suppression of this phosphorylation using an inhibitor of CK1δ or in site-specific phosphorylation-deficient mice similarly protected astrocytes from hypoxic damage. Rescue of astrocytes led to restoration of a functional retinal vasculature and lowered the hypoxic burden, thereby curtailing neovascularization and neuroretinal dysfunction. We also find that absence of astrocytic Cx43 does not affect developmental angiogenesis or neuronal function in normoxic retinas. Our in vivo work directly links phosphorylation of Cx43 to astrocytic coupling and apoptosis and ultimately to vascular regeneration in retinal ischemia. This study reveals that targeting Cx43 phosphorylation in astrocytes is a potential direction for the treatment of proliferative retinopathies.
Keywords: astrocytes; gap junctions; ischemia; neurovascular; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
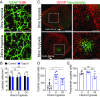

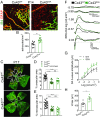
References
-
- Kempen JH, et al. Eye Diseases Prevalence Research Group The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. - PubMed
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. - PubMed
-
- Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120:2182–2194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

