Glycosylation-dependent galectin-receptor interactions promote Chlamydia trachomatis infection
- PMID: 29891717
- PMCID: PMC6042088
- DOI: 10.1073/pnas.1802188115
Glycosylation-dependent galectin-receptor interactions promote Chlamydia trachomatis infection
Abstract
Chlamydia trachomatis (Ct) constitutes the most prevalent sexually transmitted bacterium worldwide. Chlamydial infections can lead to severe clinical sequelae including pelvic inflammatory disease, ectopic pregnancy, and tubal infertility. As an obligate intracellular pathogen, Ct has evolved multiple strategies to promote adhesion and invasion of host cells, including those involving both bacterial and host glycans. Here, we show that galectin-1 (Gal1), an endogenous lectin widely expressed in female and male genital tracts, promotes Ct infection. Through glycosylation-dependent mechanisms involving recognition of bacterial glycoproteins and N-glycosylated host cell receptors, Gal1 enhanced Ct attachment to cervical epithelial cells. Exposure to Gal1, mainly in its dimeric form, facilitated bacterial entry and increased the number of infected cells by favoring Ct-Ct and Ct-host cell interactions. These effects were substantiated in vivo in mice lacking Gal1 or complex β1-6-branched N-glycans. Thus, disrupting Gal1-N-glycan interactions may limit the severity of chlamydial infection by inhibiting bacterial invasion of host cells.
Keywords: Chlamydia trachomatis; galectin-1; glycosylation; host–pathogen interactions; sexually transmitted diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
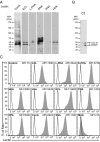
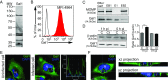
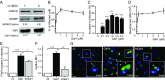


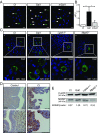
References
-
- World Health Organization 2011. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: Methods and results used by WHO to generate 2005 estimate (WHO, Geneva), pp 1–38.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

