Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis
- PMID: 29891719
- PMCID: PMC6042106
- DOI: 10.1073/pnas.1806973115
Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis
Abstract
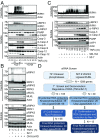
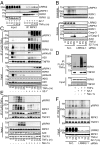
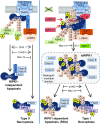
Stimulation of cells with TNFα can promote distinct cell death pathways, including RIPK1-independent apoptosis, necroptosis, and RIPK1-dependent apoptosis (RDA)-the latter of which we still know little about. Here we show that RDA involves the rapid formation of a distinct detergent-insoluble, highly ubiquitinated, and activated RIPK1 pool, termed "iuRIPK1." iuRIPK1 forms after RIPK1 activation in TNF-receptor-associated complex I, and before cytosolic complex II formation and caspase activation. To identify regulators of iuRIPK1 formation and RIPK1 activation in RDA, we conducted a targeted siRNA screen of 1,288 genes. We found that NEK1, whose loss-of-function mutations have been identified in 3% of ALS patients, binds to activated RIPK1 and restricts RDA by negatively regulating formation of iuRIPK1, while LRRK2, a kinase implicated in Parkinson's disease, promotes RIPK1 activation and association with complex I in RDA. Further, the E3 ligases APC11 and c-Cbl promote RDA, and c-Cbl is recruited to complex I in RDA, where it promotes prodeath K63-ubiquitination of RIPK1 to lead to iuRIPK1 formation. Finally, we show that two different modes of necroptosis induction by TNFα exist which are differentially regulated by iuRIPK1 formation. Overall, this work reveals a distinct mechanism of RIPK1 activation that mediates the signaling mechanism of RDA as well as a type of necroptosis.
Keywords: RIPK1; TNF; apoptosis; necroptosis; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
-
- Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2018;101:26–32. - PubMed
-
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. - PubMed
-
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

