Aptamer-Based Biosensors for Antibiotic Detection: A Review
- PMID: 29891818
- PMCID: PMC6023021
- DOI: 10.3390/bios8020054
Aptamer-Based Biosensors for Antibiotic Detection: A Review
Abstract
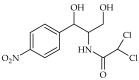
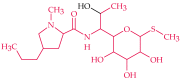
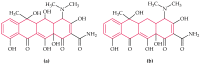
Antibiotic resistance and, accordingly, their pollution because of uncontrolled usage has emerged as a serious problem in recent years. Hence, there is an increased demand to develop robust, easy, and sensitive methods for rapid evaluation of antibiotics and their residues. Among different analytical methods, the aptamer-based biosensors (aptasensors) have attracted considerable attention because of good selectivity, specificity, and sensitivity. This review gives an overview about recently-developed aptasensors for antibiotic detection. The use of various aptamer assays to determine different groups of antibiotics, like β-lactams, aminoglycosides, anthracyclines, chloramphenicol, (fluoro)quinolones, lincosamide, tetracyclines, and sulfonamides are presented in this paper.
Keywords: ampicillin; antibiotic; aptamer; aptasensor; biosensor; chloramphenicol; ciprofloxacin; danofloxacin; daunomycin; enrofloxacin; gentamicin; kanamycin; lincomycin; neomycin; ofloxacin; oxytetracycline; penicillin; streptomycin; sulfadimethoxine; tetracycline; tobramycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

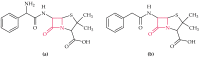



References
-
- Centers for Disease Control and Prevention, Office of Infectious Antibiotic Resistance Threats in the United States. [(accessed on 17 May 2018)]; Available online: http://www.cdc.gov/drugresistance/
-
- Gualerzi C.O., Brandi L., Fabbretti A., Pon C.L., editors. Antibiotics: Targets, Mechanisms and Resistance. Wiley-VCH Verlag; Weinheim, Germany: 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

