Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions
- PMID: 29891824
- PMCID: PMC6100375
- DOI: 10.3390/molecules23061416
Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions
Abstract
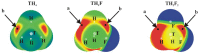
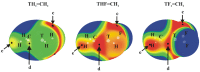
The σ-hole tetrel bonds formed by a tetravalent molecule are compared with those involving a π-hole above the tetrel atom in a trivalent bonding situation. The former are modeled by TH₄, TH₃F, and TH₂F₂ (T = Si, Ge, Sn) and the latter by TH₂=CH₂, THF=CH₂, and TF₂=CH₂, all paired with NH₃ as Lewis base. The latter π-bonded complexes are considerably more strongly bound, despite the near equivalence of the σ and π-hole intensities. The larger binding energies of the π-dimers are attributed to greater electrostatic attraction and orbital interaction. Each progressive replacement of H by F increases the strength of the tetrel bond, whether σ or π. The magnitudes of the maxima of the molecular electrostatic potential in the two types of systems are not good indicators of either the interaction energy or even the full Coulombic energy. The geometry of the Lewis acid is significantly distorted by the formation of the dimer, more so in the case of the σ-bonded complexes, and this deformation intensifies the σ and π holes.
Keywords: AIM; DFT; MEP; MP2; NBO.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence.Molecules. 2018 Oct 15;23(10):2642. doi: 10.3390/molecules23102642. Molecules. 2018. PMID: 30326582 Free PMC article.
-
Comparison of σ-/π-Hole Tetrel Bonds between TH3 F/F2 TO and H2 CX (X=O, S, Se).Chemphyschem. 2019 Feb 18;20(4):627-635. doi: 10.1002/cphc.201800990. Epub 2019 Feb 5. Chemphyschem. 2019. PMID: 30610760
-
Comparison of σ-Hole and π-Hole Tetrel Bonds Formed by Pyrazine and 1,4-Dicyanobenzene: The Interplay between Anion-π and Tetrel Bonds.Chemphyschem. 2017 Sep 20;18(18):2442-2450. doi: 10.1002/cphc.201700660. Epub 2017 Aug 11. Chemphyschem. 2017. PMID: 28708276
-
Yet another perspective on hole interactions.Phys Chem Chem Phys. 2021 Sep 22;23(36):19948-19963. doi: 10.1039/d1cp03533a. Phys Chem Chem Phys. 2021. PMID: 34514473 Review.
-
Electrostatics and Polarization in σ- and π-Hole Noncovalent Interactions: An Overview.Chemphyschem. 2020 Apr 2;21(7):579-588. doi: 10.1002/cphc.201900968. Epub 2020 Jan 16. Chemphyschem. 2020. PMID: 31733136 Review.
Cited by
-
Classification of So-Called Non-Covalent Interactions Based on VSEPR Model.Molecules. 2021 Aug 15;26(16):4939. doi: 10.3390/molecules26164939. Molecules. 2021. PMID: 34443526 Free PMC article. Review.
-
Can We Merge the Weak and Strong Tetrel Bonds? Electronic Features of Tetrahedral Molecules Interacted with Halide Anions.Molecules. 2022 Aug 24;27(17):5411. doi: 10.3390/molecules27175411. Molecules. 2022. PMID: 36080180 Free PMC article.
-
Dual Geometry Schemes in Tetrel Bonds: Complexes between TF₄ (T = Si, Ge, Sn) and Pyridine Derivatives.Molecules. 2019 Jan 21;24(2):376. doi: 10.3390/molecules24020376. Molecules. 2019. PMID: 30669688 Free PMC article.
-
σ-Hole, lone-pair-hole, and π-hole site-based interactions in aerogen-comprising complexes: a comparative study.RSC Adv. 2024 Jul 15;14(31):22408-22417. doi: 10.1039/d4ra03614j. eCollection 2024 Jul 12. RSC Adv. 2024. PMID: 39010916 Free PMC article.
-
Calculation of VS,max and Its Use as a Descriptor for the Theoretical Calculation of pKa Values for Carboxylic Acids.Molecules. 2018 Dec 26;24(1):79. doi: 10.3390/molecules24010079. Molecules. 2018. PMID: 30587832 Free PMC article.
References
-
- Desiraju G.R., Ho P.S., Kloo L., Legon A.C., Marquardt R., Metrangolo P., Politzer P., Resnati G., Rissanen K. Definition of the halogen bond (IUPAC recommendations 2013) Pure Appl. Chem. 2013;85:1711–1713. doi: 10.1351/PAC-REC-12-05-10. - DOI
-
- Esrafili M.D., Mohammadian-Sabet F. Homonuclear chalcogen-chalcogen bond interactions in complexes pairing YO3 and YHX molecules (Y=S, Se; X=H, Cl, Br, CCH, NC, OH, OCH3): Influence of substitution and cooperativity. Int. J. Quantum Chem. 2016;116:529–536. doi: 10.1002/qua.25076. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

