Fas-L promotes the stem cell potency of adipose-derived mesenchymal cells
- PMID: 29891848
- PMCID: PMC5995957
- DOI: 10.1038/s41419-018-0702-y
Fas-L promotes the stem cell potency of adipose-derived mesenchymal cells
Abstract
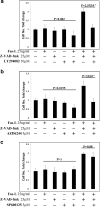
Fas-L is a TNF family member known to trigger cell death. It has recently become evident that Fas-L can transduce also non-apoptotic signals. Mesenchymal stem cells (MSCs) are multipotent cells that are derived from various adult tissues. Although MSCs from different tissues display common properties they also display tissue-specific characteristics. Previous works have demonstrated massive apoptosis following Fas-L treatment of bone marrow-derived MSCs both in vitro and following their administration in vivo. We therefore set to examine Fas-L-induced responses in adipose-derived stem cells (ASCs). Human ASCs were isolated from lipoaspirates and their reactivity to Fas-L treatment was examined. ASCs responded to Fas-L by simultaneous apoptosis and proliferation, which yielded a net doubling of cell quantities and a phenotypic shift, including reduced expression of CD105 and increased expression of CD73, in association with increased bone differentiation potential. Treatment of freshly isolated ASCs led to an increase in large colony forming unit fibroblasts, likely produced by early stem cell progenitor cells. Fas-L-induced apoptosis and proliferation signaling were found to be independent as caspase inhibition attenuated Fas-L-induced apoptosis without impacting proliferation, whereas inhibition of PI3K and MEK, but not of JNK, attenuated Fas-L-dependent proliferation, but not apoptosis. Thus, Fas-L signaling in ASCs leads to their expansion and phenotypic shift toward a more potent stem cell state. We speculate that these reactions ensure the survival of ASC progenitor cells encountering Fas-L-enriched environments during tissue damage and inflammation and may also enhance ASC survival following their administration in vivo.
Conflict of interest statement
The work was funded by Cellect Biotherapeutics Ltd.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

