Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells
- PMID: 29891854
- PMCID: PMC5995871
- DOI: 10.1038/s41598-018-27184-9
Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells
Erratum in
-
Author Correction: Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells.Sci Rep. 2020 Jul 29;10(1):13049. doi: 10.1038/s41598-020-69834-x. Sci Rep. 2020. PMID: 32724090 Free PMC article.
Abstract
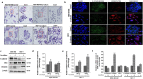
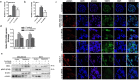
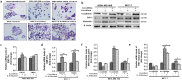
The tumour microenvironment is a key regulators of tumour progression through the secretion of growth factors that activate epithelial-mesenchymal transition (EMT). Induction of EMT is a key step for transition from a benign state to a metastatic tumour. Adipose tissue forms a bulk portion of the breast cancer microenvironment, emerging evidence indicates the potential for adipocytes to influence tumour progression through the secretion of adipokines that can induce EMT. The molecular mechanisms underlying how adipocytes enhance breast cancer progression is largely unknown. We hypothesized that paracrine signalling by adipocytes can activate EMT and results in increased migration and invasion characteristics of breast cancer cells. We found that IL-6 secreted by adipocytes induce EMT in breast cancer cells. The effect of IL-6 expression on EMT is mediated through activation of the signal transducer and activated of transcription 3 (STAT3). Blocking of IL-6 signalling in breast cancer cells and adipocytes, decreased proliferation, migration and invasion capabilities and altered the expression of genes regulating EMT. Together, our results suggest that matured human adipocytes can enhance the aggressive behaviour of breast cancer cells and induce an EMT-phenotype through paracrine IL-6/STAT3 signalling.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Niclosamide reverses adipocyte induced epithelial-mesenchymal transition in breast cancer cells via suppression of the interleukin-6/STAT3 signalling axis.Sci Rep. 2019 Aug 5;9(1):11336. doi: 10.1038/s41598-019-47707-2. Sci Rep. 2019. PMID: 31383893 Free PMC article.
-
Paracrine IL-6 signaling mediates the effects of pancreatic stellate cells on epithelial-mesenchymal transition via Stat3/Nrf2 pathway in pancreatic cancer cells.Biochim Biophys Acta Gen Subj. 2017 Feb;1861(2):296-306. doi: 10.1016/j.bbagen.2016.10.006. Epub 2016 Oct 14. Biochim Biophys Acta Gen Subj. 2017. PMID: 27750041
-
Cancer-associated adipocyte-derived G-CSF promotes breast cancer malignancy via Stat3 signaling.J Mol Cell Biol. 2020 Sep 1;12(9):723-737. doi: 10.1093/jmcb/mjaa016. J Mol Cell Biol. 2020. PMID: 32242230 Free PMC article.
-
Role of STAT3 in cancer cell epithelial‑mesenchymal transition (Review).Int J Oncol. 2024 May;64(5):48. doi: 10.3892/ijo.2024.5636. Epub 2024 Mar 15. Int J Oncol. 2024. PMID: 38488027 Free PMC article. Review.
-
Cancer-associated adipocytes: emerging supporters in breast cancer.J Exp Clin Cancer Res. 2020 Aug 12;39(1):156. doi: 10.1186/s13046-020-01666-z. J Exp Clin Cancer Res. 2020. PMID: 32787888 Free PMC article. Review.
Cited by
-
Recent Advances in the Aging Microenvironment of Breast Cancer.Cancers (Basel). 2022 Oct 12;14(20):4990. doi: 10.3390/cancers14204990. Cancers (Basel). 2022. PMID: 36291773 Free PMC article. Review.
-
The Role of Adipokines and Bone Marrow Adipocytes in Breast Cancer Bone Metastasis.Int J Mol Sci. 2020 Jul 14;21(14):4967. doi: 10.3390/ijms21144967. Int J Mol Sci. 2020. PMID: 32674405 Free PMC article. Review.
-
Expression of Protein 4.1 Family in Breast Cancer: Database Mining for 4.1 Family Members in Malignancies.Med Sci Monit. 2019 May 7;25:3374-3389. doi: 10.12659/MSM.914085. Med Sci Monit. 2019. PMID: 31063460 Free PMC article.
-
STAT proteins in cancer: orchestration of metabolism.Nat Rev Cancer. 2023 Mar;23(3):115-134. doi: 10.1038/s41568-022-00537-3. Epub 2023 Jan 3. Nat Rev Cancer. 2023. PMID: 36596870 Review.
-
Tumor-produced immune regulatory factors as a therapeutic target in cancer treatment.Front Immunol. 2024 Aug 14;15:1416458. doi: 10.3389/fimmu.2024.1416458. eCollection 2024. Front Immunol. 2024. PMID: 39206193 Free PMC article. Review. No abstract available.
References
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J Clin. 66, 7–30, 10.3322/caac.21332, Epub22016 Jan 21337 (2016). - PubMed
-
- Yang, J. & Weinberg, R. A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 14, 818–829, doi: 810.1016/j.devcel.2008.1005.1009 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

