Broad phenotypes in heterozygous NR5A1 46,XY patients with a disorder of sex development: an oligogenic origin?
- PMID: 29891883
- PMCID: PMC6117353
- DOI: 10.1038/s41431-018-0202-7
Broad phenotypes in heterozygous NR5A1 46,XY patients with a disorder of sex development: an oligogenic origin?
Abstract
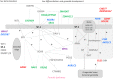
SF-1/NR5A1 is a transcriptional regulator of adrenal and gonadal development. NR5A1 disease-causing variants cause disorders of sex development (DSD) and adrenal failure, but most affected individuals show a broad DSD/reproductive phenotype only. Most NR5A1 variants show in vitro pathogenic effects, but not when tested in heterozygote state together with wild-type NR5A1 as usually seen in patients. Thus, the genotype-phenotype correlation for NR5A1 variants remains an unsolved question. We analyzed heterozygous 46,XY SF-1/NR5A1 patients by whole exome sequencing and used an algorithm for data analysis based on selected project-specific DSD- and SF-1-related genes. The variants detected were evaluated for their significance in literature, databases and checked in silico using webtools. We identified 19 potentially deleterious variants (one to seven per patient) in 18 genes in four 46,XY DSD subjects carrying heterozygous NR5A1 disease-causing variants. We constructed a scheme of all these hits within the landscape of currently known genes involved in male sex determination and differentiation. Our results suggest that the broad phenotype in these heterozygous NR5A1 46,XY DSD subjects may well be explained by an oligogenic mode of inheritance, in which multiple hits, individually non-deleterious, may contribute to a DSD phenotype unique to each heterozygous SF-1/NR5A1 individual.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6:1249–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

