Clinical significance of CCR7+CD8+ T cells in kidney transplant recipients with allograft rejection
- PMID: 29891963
- PMCID: PMC5995850
- DOI: 10.1038/s41598-018-27141-6
Clinical significance of CCR7+CD8+ T cells in kidney transplant recipients with allograft rejection
Abstract
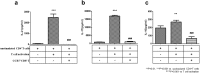
The regulatory function of CCR7+CD8+ T cells against effector T-cells involved in T-cell mediated rejection (TCMR) in kidney transplant recipients was investigated. In vitro experiments explored the ability of CCR7+CD8+ T cells to suppress T-cell proliferation under T-cell activation conditions or during coculture with human renal proximal tubular epithelial cells (HRPTEpiC). In an ex vivo experiment, the proportion of CCR7+/CD8+, FOXP3+/CCR7+CD8+ T and effector T-cell subsets were compared between the normal biopsy control (NC, n = 17) and TCMR group (n = 17). The CCR7+CD8+ T cells significantly suppressed the proliferation of CD4+ T cells and significantly decreased the proportion of IFN-γ+ and IL-17+/CD4+ T cells and inflammatory cytokine levels (all p < 0.05). After coculturing with HRPTEpiC, CCR7+CD8+ T cells also suppressed T-cell differentiation into IL-2+, IFN-γ+, and IL-17+/CD4+ T cells (all p < 0.05). The TCMR group had significantly fewer CCR7+/CD8+ and FOXP3+/CCR7+CD8+ T in comparison with the NC group, but the proportions of all three effector T-cell subsets were increased in the TCMR group (all p < 0.05). The proportion of CCR7+/CD8+ T was inversely correlated with those of effector T-cell subsets. The results indicate that CCR7+CD8+ T cells may regulate effector T-cells involved in TCMR in an in vitro and in an ex vivo transplant model.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Gill RG. An orchestrated dance between differing regulatory T cell phenotypes in allograft tolerance. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1945–1946. doi: 10.1111/ajt.12336. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

