Sensing Cell-Culture Assays with Low-Cost Circuitry
- PMID: 29892009
- PMCID: PMC5995807
- DOI: 10.1038/s41598-018-27295-3
Sensing Cell-Culture Assays with Low-Cost Circuitry
Abstract
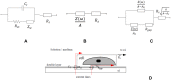
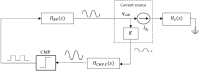
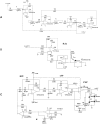
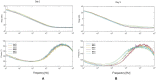
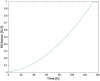
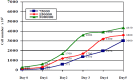
An alternative approach for cell-culture end-point protocols is proposed herein. This new technique is suitable for real-time remote sensing. It is based on Electrical Cell-substrate Impedance Spectroscopy (ECIS) and employs the Oscillation-Based Test (OBT) method. Simple and straightforward circuit blocks form the basis of the proposed measurement system. Oscillation parameters - frequency and amplitude - constitute the outcome, directly correlated with the culture status. A user can remotely track the evolution of cell cultures in real time over the complete experiment through a web tool continuously displaying the acquired data. Experiments carried out with commercial electrodes and a well-established cell line (AA8) are described, obtaining the cell number in real time from growth assays. The electrodes have been electrically characterized along the design flow in order to predict the system performance and the sensitivity curves. Curves for 1-week cell growth are reported. The obtained experimental results validate the proposed OBT for cell-culture characterization. Furthermore, the proposed electrode model provides a good approximation for the cell number and the time evolution of the studied cultures.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Freshney, R. I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 7th Edition, Wiley-Blackwell. (2016).
-
- Reitinger S, Wissenwasser J, Kapferer W, Heer R, Lepperdinger G. Electric impedance sensing in cell-substrates for rapid and selective multipotential differentiation capacity monitoring of human mesenchymal stem cells. Biosensors and Bioelectronics. 2012;34(no 1):63–69. doi: 10.1016/j.bios.2012.01.013. - DOI - PubMed
-
- Yeh, C., To, P. & Lin, Y. Using the micro-array electrode chip and AC signals to generate the electric field effect on cell migration. Sensors and Actuators B: Chemical (2012).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

