Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection
- PMID: 29892063
- PMCID: PMC6092240
- DOI: 10.1038/s41591-018-0026-6
Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection
Abstract
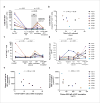
Antiretroviral therapy during the earliest stage of acute HIV infection (Fiebig I) might minimize establishment of a latent HIV reservoir and thereby facilitate viremic control after analytical treatment interruption. We show that 8 participants, who initiated treatment during Fiebig I and were treated for a median of 2.8 years, all experienced rapid viral load rebound following analytical treatment interruption, indicating that additional strategies are required to control or eradicate HIV.
Conflict of interest statement
TC has received a speaker’s fee from Gilead. JA has received honorarium for participating in advisory meetings from Merck, Roche, AbbVie and ViiV Healthcare. All other authors declare no competing financial interests.
Figures

References
MANUSCRIPT REFERENCES
-
- Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science (New York, NY) 1997;278:1295–1300. - PubMed
ONLINE METHODS REFERENCES
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

