Relevance of Aβ42/40 Ratio for Detection of Alzheimer Disease Pathology in Clinical Routine: The PLMR Scale
- PMID: 29892221
- PMCID: PMC5985301
- DOI: 10.3389/fnagi.2018.00138
Relevance of Aβ42/40 Ratio for Detection of Alzheimer Disease Pathology in Clinical Routine: The PLMR Scale
Abstract
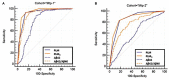
Background: Cerebrospinal fluid (CSF) biomarkers (Aβ peptides and tau proteins) improved the diagnosis of Alzheimer's disease (AD) in research and clinical settings. We previously described the PLM-scale (Paris-Lille-Montpellier study), which combines Aβ42, tau, and phosphorylated ptau(181) biomarkers in an easy to use and clinically relevant way. The purpose of this work is to evaluate an optimized PLMR-scale (PLM ratio scale) that now includes the Aβ42/Aβ40 ratio to detect AD versus non-AD (NAD) participants in clinical routine of memory centers. Methods: Both scales were compared using 904 participants with cognitive impairment recruited from two independent cohorts (Mtp-1 and Mtp-2). The CSF Aβ42/Aβ40 ratio was measured systematically in Mtp-1, and only on biologically discordant cases in Mtp-2. Two different ELISA kit providers were also employed. The distribution of AD and NAD patients and the discrepancies of biomarker profiles were computed. Receiver Operating Characteristic curves were used to represent clinical sensitivity and specificity for AD detection. The classification of patients with the net reclassification index (NRI) was also evaluated. Results: Nine hundred and four participants (342 AD and 562 NAD) were studied; 400 in Mtp-1 and 504 in Mtp-2. For AD patients, the mean CSF Aβ42 and CSF Aβ42/40 ratio was 553 ± 216 pg/mL and 0.069 ± 0.022 pg/mL in Mtp-1 and 702 ± 335 pg/mL and 0.045 ± 0.020 pg/mL in Mtp-2. The distribution of AD and NAD differed between the PLM and the PLMR scales (p < 0.0001). The percentage AD well-classified (class 3) increased with PLMR from 38 to 83% in Mpt-1 and from 33 to 53% in Mpt-2. A sharp reduction of the discordant profiles going from 34 to 16.3% and from 37.5 to 19.8%, for Mtp-1 and Mtp-2 respectively, was also observed. The AUC of the PLMR scale was 0.94 in Mtp-1 and 0.87 in Mtp-2. In both cohorts, the PLMR outperformed CSF Aβ42 or Aβ42/40 ratio. The diagnostic performance was improved with the PLMR with an NRI equal to 44.3% in Mtp-1 and 28.8% in Mtp-2. Conclusion: The integration of the Aβ42/Aβ40 ratio in the PLMR scale resulted in an easy-to-use tool which reduced the discrepancies in biologically doubtful cases and increased the confidence in the diagnosis in memory center.
Keywords: Alzheimer’s disease; biomarkers; cerebrospinal fluid (CSF); screening scale.
Figures

Similar articles
-
Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study.Alzheimers Res Ther. 2015 Jun 1;7(1):30. doi: 10.1186/s13195-015-0114-5. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 26034513 Free PMC article.
-
Cerebrospinal Fluid Aβ40 Improves the Interpretation of Aβ42 Concentration for Diagnosing Alzheimer's Disease.Front Neurol. 2015 Nov 27;6:247. doi: 10.3389/fneur.2015.00247. eCollection 2015. Front Neurol. 2015. PMID: 26640457 Free PMC article.
-
Additional use of Aβ₄₂/Aβ₄₀ ratio with cerebrospinal fluid biomarkers P-tau and Aβ₄₂ increases the level of evidence of Alzheimer's disease pathophysiological process in routine practice.J Alzheimers Dis. 2014;41(2):377-86. doi: 10.3233/JAD-131838. J Alzheimers Dis. 2014. PMID: 24614902
-
Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's Disease.Alzheimers Res Ther. 2019 Apr 22;11(1):34. doi: 10.1186/s13195-019-0485-0. Alzheimers Res Ther. 2019. PMID: 31010420 Free PMC article. Review.
-
Fluid biomarker-based molecular phenotyping of Alzheimer's disease patients in research and clinical settings.Prog Mol Biol Transl Sci. 2019;168:3-23. doi: 10.1016/bs.pmbts.2019.07.006. Epub 2019 Jul 24. Prog Mol Biol Transl Sci. 2019. PMID: 31699324 Review.
Cited by
-
Origins of Beta Amyloid Differ Between Vascular Amyloid Deposition and Parenchymal Amyloid Plaques in the Spinal Cord of a Mouse Model of Alzheimer's Disease.Mol Neurobiol. 2020 Jan;57(1):278-289. doi: 10.1007/s12035-019-01697-4. Epub 2019 Jul 19. Mol Neurobiol. 2020. PMID: 31325023
-
Comparison of ultrasensitive and mass spectrometry quantification of blood-based amyloid biomarkers for Alzheimer's disease diagnosis in a memory clinic cohort.Alzheimers Res Ther. 2023 Feb 18;15(1):34. doi: 10.1186/s13195-023-01188-8. Alzheimers Res Ther. 2023. PMID: 36800984 Free PMC article.
-
Erlangen Score as a tool to predict progression from mild cognitive impairment to dementia in Alzheimer's disease.Alzheimers Res Ther. 2019 Jan 5;11(1):2. doi: 10.1186/s13195-018-0456-x. Alzheimers Res Ther. 2019. PMID: 30611311 Free PMC article.
-
Potential neuroprotection by Dendrobium nobile Lindl alkaloid in Alzheimer's disease models.Neural Regen Res. 2022 May;17(5):972-977. doi: 10.4103/1673-5374.324824. Neural Regen Res. 2022. PMID: 34558510 Free PMC article.
-
Cognitive Phenotyping and Interpretation of Alzheimer Blood Biomarkers.JAMA Neurol. 2025 May 1;82(5):506-515. doi: 10.1001/jamaneurol.2025.0142. JAMA Neurol. 2025. PMID: 40181683 Free PMC article.
References
-
- Del Campo M., Mollenhauer B., Bertolotto A., Engelborghs S., Hampel H., Simonsen A. H., et al. (2012). Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: an update. Biomark. Med. 6 419–430. 10.2217/bmm.12.46 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

