Acclimation and Institutionalization of the Mouse Microbiota Following Transportation
- PMID: 29892276
- PMCID: PMC5985407
- DOI: 10.3389/fmicb.2018.01085
Acclimation and Institutionalization of the Mouse Microbiota Following Transportation
Abstract
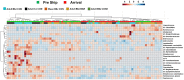
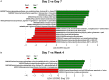
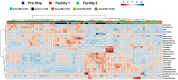
Using animal models, the gut microbiota has been shown to play a critical role in the health and disease of many organ systems. Unfortunately, animal model studies often lack reproducibility when performed at different institutions. Previous studies in our laboratory have shown that the gut microbiota of mice can vary with a number of husbandry factors leading us to speculate that differing environments may alter gut microbiota, which in turn may influence animal model phenotypes. As an extension of these studies, we hypothesized that the shipping of mice from a mouse producer to an institution will result in changes in the type, relative abundance, and functional composition of the gut microbiota. Furthermore, we hypothesized that mice will develop a microbiota unique to the institution and facility in which they are housed. To test these hypotheses, mice of two strains (C57BL/6J and BALB/cJ), two age groups (4 week and 8 week old), and originating from two types of housing (research animal facility under conventional housing and production facilities under maximum barrier housing) were obtained from The Jackson Laboratory. Fecal samples were collected the day prior to shipping, immediately upon arrival, and then on days 2, 5, 7, and weeks 2, 4, and 9 post-arrival. Following the first post-arrival fecal collection, mice were separated into 2 groups and housed at different facilities at our institution while keeping their caging, diet, and husbandry practices the same. DNA was extracted from the collected fecal pellets and 16S rRNA amplicons were sequenced in order to characterize the type and relative abundance of gut bacteria. Principal component analysis (PCA) and permutational multivariate analysis of variance (PERMANOVA) demonstrated that both the shipping and the institution and facility in which mice were housed altered the gut microbiota. Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) predicted differences in functional composition in the gut microbiota of mice based on time of acclimation.
Keywords: 16S rRNA gene sequencing; acclimation; gut microbiota (GM); mouse models; transportation.
Figures




References
-
- Aguila H., Pakes S., Lai W., Lu Y.-S. (1988). The effect of transportation stress on splenic natural killer cell activity in C57BL/6J mice. Lab. Anim. Sci. 38, 148–151. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

