The Absent in Melanoma 2-Like Receptor IFN-Inducible Protein 16 as an Inflammasome Regulator in Systemic Lupus Erythematosus: The Dark Side of Sensing Microbes
- PMID: 29892303
- PMCID: PMC5985366
- DOI: 10.3389/fimmu.2018.01180
The Absent in Melanoma 2-Like Receptor IFN-Inducible Protein 16 as an Inflammasome Regulator in Systemic Lupus Erythematosus: The Dark Side of Sensing Microbes
Abstract
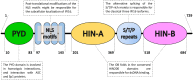
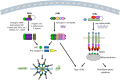
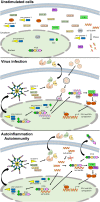
Absent in melanoma 2 (AIM2)-like receptors (ALRs) are a newly characterized class of pathogen recognition receptors (PRRs) involved in cytosolic and nuclear pathogen DNA recognition. In recent years, two ALR family members, the interferon (IFN)-inducible protein 16 (IFI16) and AIM2, have been linked to the pathogenesis of various autoimmune diseases, among which systemic lupus erythematosus (SLE) has recently gained increasing attention. SLE patients are indeed often characterized by constitutively high serum IFN levels and increased expression of IFN-stimulated genes due to an abnormal response to pathogens and/or incorrect self-DNA recognition process. Consistently, we and others have shown that IFI16 is overexpressed in a wide range of autoimmune diseases where it triggers production of specific autoantibodies. In addition, evidence from mouse models supports a model whereby ALRs are required for IFN-mediated host response to both exogenous and endogenous DNA. Following interaction with cytoplasmic or nuclear nucleic acids, ALRs can form a functional inflammasome through association with the adaptor ASC [apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD)] and with procaspase-1. Importantly, inflammasome-mediated upregulation of IL-1β and IL-18 production positively correlates with SLE disease severity. Therefore, targeting ALR sensors and their downstream pathways represents a promising alternative therapeutic approach for SLE and other systemic autoimmune diseases.
Keywords: IFN-inducible protein 16; absent in melanoma 2 (AIM2)-like receptor; inflammasome; interferon; systemic lupus erythematosus.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

