Immunometabolic Activation of Invariant Natural Killer T Cells
- PMID: 29892305
- PMCID: PMC5985373
- DOI: 10.3389/fimmu.2018.01192
Immunometabolic Activation of Invariant Natural Killer T Cells
Abstract
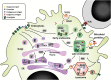
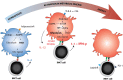
Invariant natural killer T (iNKT) cells are lipid-reactive T cells with profound immunomodulatory potential. They are unique in their restriction to lipid antigens presented in CD1d molecules, which underlies their role in lipid-driven disorders such as obesity and atherosclerosis. In this review, we discuss the contribution of iNKT cell activation to immunometabolic disease, metabolic programming of lipid antigen presentation, and immunometabolic activation of iNKT cells. First, we outline the role of iNKT cells in immunometabolic disease. Second, we discuss the effects of cellular metabolism on lipid antigen processing and presentation to iNKT cells. The synthesis and processing of glycolipids and other potential endogenous lipid antigens depends on metabolic demand and may steer iNKT cells toward adopting a Th1 or Th2 signature. Third, external signals such as toll-like receptor ligands, adipokines, and cytokines modulate antigen presentation and subsequent iNKT cell responses. Finally, we will discuss the relevance of metabolic programming of iNKT cells in human disease, focusing on their role in disorders such as obesity and atherosclerosis. The critical response to metabolic changes places iNKT cells at the helm of immunometabolic disease.
Keywords: AMPK; NKT; atherosclerosis; immunometabolism; mTOR; obesity; sphingolipid.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

