The Effect of Single and Multiple SERAT Mutants on Serine and Sulfur Metabolism
- PMID: 29892307
- PMCID: PMC5985473
- DOI: 10.3389/fpls.2018.00702
The Effect of Single and Multiple SERAT Mutants on Serine and Sulfur Metabolism
Abstract
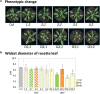
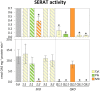
The gene family of serine acetyltransferases (SERATs) constitutes an interface between the plant pathways of serine and sulfur metabolism. SERATs provide the activated precursor, O-acetylserine for the fixation of reduced sulfur into cysteine by exchanging the serine hydroxyl moiety by a sulfhydryl moiety, and subsequently all organic compounds containing reduced sulfur moieties. We investigate here, how manipulation of the SERAT interface results in metabolic alterations upstream or downstream of this boundary and the extent to which the five SERAT isoforms exert an effect on the coupled system, respectively. Serine is synthesized through three distinct pathways while cysteine biosynthesis is distributed over the three compartments cytosol, mitochondria, and plastids. As the respective mutants are viable, all necessary metabolites can obviously cross various membrane systems to compensate what would otherwise constitute a lethal failure in cysteine biosynthesis. Furthermore, given that cysteine serves as precursor for multiple pathways, cysteine biosynthesis is highly regulated at both, the enzyme and the expression level. In this study, metabolite profiles of a mutant series of the SERAT gene family displayed that levels of the downstream metabolites in sulfur metabolism were affected in correlation with the reduction levels of SERAT activities and the growth phenotypes, while levels of the upstream metabolites in serine metabolism were unchanged in the serat mutants compared to wild-type plants. These results suggest that despite of the fact that the two metabolic pathways are directly connected, there seems to be no causal link in metabolic alterations. This might be caused by the difference of their pool sizes or the tight regulation by homeostatic mechanisms that control the metabolite concentration in plant cells. Additionally, growth conditions exerted an influence on metabolic compositions.
Keywords: O-acetylserine; cysteine; serine; serine acetyltransferase; subcellular compartment.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

