Phoretic interactions and oscillations in active suspensions of growing Escherichia coli
- PMID: 29892439
- PMCID: PMC5990789
- DOI: 10.1098/rsos.180008
Phoretic interactions and oscillations in active suspensions of growing Escherichia coli
Abstract
Bioluminescence imaging experiments were carried out to characterize spatio-temporal patterns of bacterial self-organization in active suspensions (cultures) of bioluminescent Escherichia coli and its mutants. An analysis of the effects of mutations shows that spatio-temporal patterns formed in standard microtitre plates are not related to the chemotaxis system of bacteria. In fact, these patterns are strongly dependent on the properties of mutants that characterize them as self-phoretic (non-flagellar) swimmers. In particular, the observed patterns are essentially dependent on the efficiency of proton translocation across membranes and the smoothness of the cell surface. These characteristics can be associated, respectively, with the surface activity and the phoretic mobility of a colloidal swimmer. An analysis of the experimental data together with mathematical modelling of pattern formation suggests the following: (1) pattern-forming processes can be described by Keller-Segel-type models of chemotaxis with logistic cell kinetics; (2) active cells can be seen as biochemical oscillators that exhibit phoretic drift and alignment; and (3) the spatio-temporal patterns in a suspension of growing E. coli form due to phoretic interactions between oscillating cells of high metabolic activity.
Keywords: Janus particles; bacterial chemotaxis; bacterial growth; biochemical oscillations; self-phoresis.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
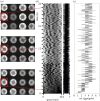

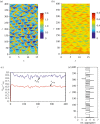
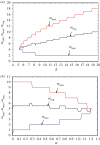


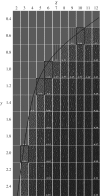
References
-
- Poon WCK. 2013. From clarkia to Escherichia and Janus: the physics of natural and synthetic active colloids. In Physics of complex colloids, vol. 184 (eds Bechinger C, Sciortino F, Ziherl P), pp. 317–386. Proceedings of the International School of Physics Enrico Fermi Amsterdam, The Netherlands: IOS Press.
-
- Zöttl A, Stark H. 2016. Emergent behavior in active colloids. J. Phys. Condens. Matter 28, 253001 (doi:10.1088/0953-8984/28/25/253001) - DOI
-
- Schwarz-Linek J, Arlt J, Jepson A, Dawson A, Vissers T, Miroli D, Pilizota T, Martinez VA, Poon WCK. 2016. Escherichia coli as a model active colloid: a practical introduction. Colloids Surf. B Biointerfaces 137, 2–16. (doi:10.1016/j.colsurfb.2015.07.048), - DOI - PubMed
-
- Moran JL, Posner JD. 2017. Phoretic self-propulsion. Annu. Rev. Fluid Mech. 49, 511–540. (doi:10.1146/annurev-fluid-122414-034456) - DOI
-
- Berg HC. 2000. Motile behavior of bacteria. Phys. Today 53, 24–29. (doi:10.1063/1.882934) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
