Transcriptional cofactors Ski and SnoN are major regulators of the TGF-β/Smad signaling pathway in health and disease
- PMID: 29892481
- PMCID: PMC5992185
- DOI: 10.1038/s41392-018-0015-8
Transcriptional cofactors Ski and SnoN are major regulators of the TGF-β/Smad signaling pathway in health and disease
Abstract
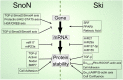
The transforming growth factor-β (TGF-β) family plays major pleiotropic roles by regulating many physiological processes in development and tissue homeostasis. The TGF-β signaling pathway outcome relies on the control of the spatial and temporal expression of >500 genes, which depend on the functions of the Smad protein along with those of diverse modulators of this signaling pathway, such as transcriptional factors and cofactors. Ski (Sloan-Kettering Institute) and SnoN (Ski novel) are Smad-interacting proteins that negatively regulate the TGF-β signaling pathway by disrupting the formation of R-Smad/Smad4 complexes, as well as by inhibiting Smad association with the p300/CBP coactivators. The Ski and SnoN transcriptional cofactors recruit diverse corepressors and histone deacetylases to repress gene transcription. The TGF-β/Smad pathway and coregulators Ski and SnoN clearly regulate each other through several positive and negative feedback mechanisms. Thus, these cross-regulatory processes finely modify the TGF-β signaling outcome as they control the magnitude and duration of the TGF-β signals. As a result, any alteration in these regulatory mechanisms may lead to disease development. Therefore, the design of targeted therapies to exert tight control of the levels of negative modulators of the TGF-β pathway, such as Ski and SnoN, is critical to restore cell homeostasis under the specific pathological conditions in which these cofactors are deregulated, such as fibrosis and cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Downregulation of SnoN oncoprotein induced by antibiotics anisomycin and puromycin positively regulates transforming growth factor-β signals.Biochim Biophys Acta. 2013 Nov;1830(11):5049-58. doi: 10.1016/j.bbagen.2013.07.006. Epub 2013 Jul 18. Biochim Biophys Acta. 2013. PMID: 23872350
-
Up-regulated transcriptional repressors SnoN and Ski bind Smad proteins to antagonize transforming growth factor-beta signals during liver regeneration.J Biol Chem. 2002 Aug 9;277(32):28483-90. doi: 10.1074/jbc.M202403200. Epub 2002 May 21. J Biol Chem. 2002. PMID: 12023281
-
Downregulation of Smad transcriptional corepressors SnoN and Ski in the fibrotic kidney: an amplification mechanism for TGF-beta1 signaling.J Am Soc Nephrol. 2003 Dec;14(12):3167-77. doi: 10.1097/01.asn.0000099373.33259.b2. J Am Soc Nephrol. 2003. PMID: 14638915
-
SnoN in TGF-beta signaling and cancer biology.Curr Mol Med. 2008 Jun;8(4):319-28. doi: 10.2174/156652408784533797. Curr Mol Med. 2008. PMID: 18537639 Review.
-
Smad transcriptional corepressors in TGF beta family signaling.Curr Top Microbiol Immunol. 2001;254:145-64. Curr Top Microbiol Immunol. 2001. PMID: 11190572 Review.
Cited by
-
Regulation of transforming growth factor-β signaling as a therapeutic approach to treating colorectal cancer.World J Gastroenterol. 2022 Sep 7;28(33):4744-4761. doi: 10.3748/wjg.v28.i33.4744. World J Gastroenterol. 2022. PMID: 36156927 Free PMC article. Review.
-
Interferon partly dictates a divergent transcriptional response in poxvirus-infected and bystander inflammatory monocytes.Cell Rep. 2022 Nov 22;41(8):111676. doi: 10.1016/j.celrep.2022.111676. Cell Rep. 2022. PMID: 36417857 Free PMC article.
-
The Oncoprotein SKI Acts as A Suppressor of NK Cell-Mediated Immunosurveillance in PDAC.Cancers (Basel). 2020 Oct 3;12(10):2857. doi: 10.3390/cancers12102857. Cancers (Basel). 2020. PMID: 33023028 Free PMC article.
-
Organic phosphate but not inorganic phosphate regulates Fgf23 expression through MAPK and TGF-ꞵ signaling.iScience. 2024 Mar 29;27(6):109625. doi: 10.1016/j.isci.2024.109625. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883842 Free PMC article.
-
Hitting the Target! Challenges and Opportunities for TGF-β Inhibition for the Treatment of Cardiac fibrosis.Pharmaceuticals (Basel). 2024 Feb 20;17(3):267. doi: 10.3390/ph17030267. Pharmaceuticals (Basel). 2024. PMID: 38543053 Free PMC article. Review.
References
-
- Moses, H. L., Roberts, A. B. The discovery of TGF-β: A historical perspective. In: Derynck R., Miyazono K. (eds). The TGF-β Family, vol. 50. Cold Spring Harbor Laboratory Press: New York, 2008, pp 1–28.
-
- Wrana, J. L., Ozdamar, B., Le Roy, C., Benchabane, H. Signaling Receptors of the TGF β family. In: Derynck R., Miyazono K. (eds). The TGF-β Family, vol. 50. Cold Spring Harbor Laboratory Press: New York, 2008, pp 151–178.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

