Metal-binding polymorphism in late embryogenesis abundant protein AtLEA4-5, an intrinsically disordered protein
- PMID: 29892507
- PMCID: PMC5994335
- DOI: 10.7717/peerj.4930
Metal-binding polymorphism in late embryogenesis abundant protein AtLEA4-5, an intrinsically disordered protein
Abstract
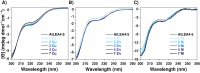
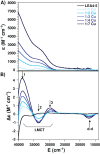
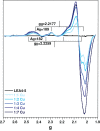
Late embryogenesis abundant (LEA) proteins accumulate in plants during adverse conditions and their main attributed function is to confer tolerance to stress. One of the deleterious effects of the adverse environment is the accumulation of metal ions to levels that generate reactive oxygen species, compromising the survival of cells. AtLEA4-5, a member of group 4 of LEAs in Arabidopsis, is an intrinsically disordered protein. It has been shown that their N-terminal region is able to undergo transitions to partially folded states and prevent the inactivation of enzymes. We have characterized metal ion binding to AtLEA4-5 by circular dichroism, electronic absorbance spectroscopy (UV-vis), electron paramagnetic resonance, dynamic light scattering, and isothermal titration calorimetry. The data shows that AtLEA4-5 contains a single binding site for Ni(II), while Zn(II) and Cu(II) have multiple binding sites and promote oligomerization. The Cu(II) interacts preferentially with histidine residues mostly located in the C-terminal region with moderate affinity and different coordination modes. These results and the lack of a stable secondary structure formation indicate that an ensemble of conformations remains accessible to the metal for binding, suggesting the formation of a fuzzy complex. Our results support the multifunctionality of LEA proteins and suggest that the C-terminal region of AtLEA4-5 could be responsible for antioxidant activity, scavenging metal ions under stress conditions while the N-terminal could function as a chaperone.
Keywords: Fuzzy complex; Intrinsically disordered proteins; Metal binding; Protein self-assembly.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Bernarducci E, Schwindinger WF, Hughey JL, Krogh-Jespersen K, Schugar HJ. Electronic spectra of copper(II)-imidazole and copper(II)-pyrazole chromophores. Journal of the American Chemical Society. 1981;103(7):1686–1691. doi: 10.1021/ja00397a017. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

