NemaFlex: a microfluidics-based technology for standardized measurement of muscular strength of C. elegans
- PMID: 29892747
- PMCID: PMC6057834
- DOI: 10.1039/c8lc00103k
NemaFlex: a microfluidics-based technology for standardized measurement of muscular strength of C. elegans
Abstract
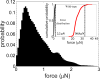
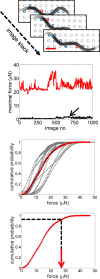
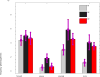
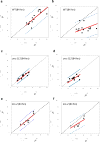
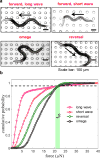
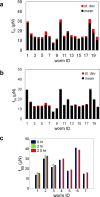
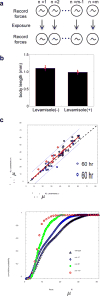
Muscle strength is a functional measure of quality of life in humans. Declines in muscle strength are manifested in diseases as well as during inactivity, aging, and space travel. With conserved muscle biology, the simple genetic model C. elegans is a high throughput platform in which to identify molecular mechanisms causing muscle strength loss and to develop interventions based on diet, exercise, and drugs. In the clinic, standardized strength measures are essential to quantitate changes in patients; however, analogous standards have not been recapitulated in the C. elegans model since force generation fluctuates based on animal behavior and locomotion. Here, we report a microfluidics-based system for strength measurement that we call 'NemaFlex', based on pillar deflection as the nematode crawls through a forest of pillars. We have optimized the micropillar forest design and identified robust measurement conditions that yield a measure of strength that is independent of behavior and gait. Validation studies using a muscle contracting agent and mutants confirm that NemaFlex can reliably score muscular strength in C. elegans. Additionally, we report a scaling factor to account for animal size that is consistent with a biomechanics model and enables comparative strength studies of mutants. Taken together, our findings anchor NemaFlex for applications in genetic and drug screens, for defining molecular and cellular circuits of neuromuscular function, and for dissection of degenerative processes in disuse, aging, and disease.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Muscle strength deficiency and mitochondrial dysfunction in a muscular dystrophy model of Caenorhabditis elegans and its functional response to drugs.Dis Model Mech. 2018 Dec 4;11(12):dmm036137. doi: 10.1242/dmm.036137. Dis Model Mech. 2018. PMID: 30396907 Free PMC article.
-
Investigating the correlation of muscle function tests and sarcomere organization in C. elegans.Skelet Muscle. 2021 Aug 13;11(1):20. doi: 10.1186/s13395-021-00275-4. Skelet Muscle. 2021. PMID: 34389048 Free PMC article.
-
On-chip analysis of C. elegans muscular forces and locomotion patterns in microstructured environments.Lab Chip. 2013 May 7;13(9):1699-707. doi: 10.1039/c3lc41403e. Lab Chip. 2013. PMID: 23511608
-
Microfluidic Technologies for High Throughput Screening Through Sorting and On-Chip Culture of C. elegans.Molecules. 2019 Nov 25;24(23):4292. doi: 10.3390/molecules24234292. Molecules. 2019. PMID: 31775328 Free PMC article. Review.
-
Studying Parkinson's disease using Caenorhabditis elegans models in microfluidic devices.Integr Biol (Camb). 2019 May 1;11(5):186-207. doi: 10.1093/intbio/zyz017. Integr Biol (Camb). 2019. PMID: 31251339 Review.
Cited by
-
A Compact Imaging Platform for Conducting C. elegans Phenotypic Assays on Earth and in Spaceflight.Life (Basel). 2023 Jan 10;13(1):200. doi: 10.3390/life13010200. Life (Basel). 2023. PMID: 36676149 Free PMC article.
-
Optimized criteria for locomotion-based healthspan evaluation in C. elegans using the WorMotel system.PLoS One. 2020 Mar 3;15(3):e0229583. doi: 10.1371/journal.pone.0229583. eCollection 2020. PLoS One. 2020. PMID: 32126105 Free PMC article.
-
A review: oxidative stress in skeletal muscle and the non-coding RNAs behind it.Mol Cell Biochem. 2025 Jun 30. doi: 10.1007/s11010-025-05339-3. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40588664
-
Digging deeper: methodologies for high-content phenotyping in Caenorhabditis elegans.Lab Anim (NY). 2019 Jul;48(7):207-216. doi: 10.1038/s41684-019-0326-6. Epub 2019 Jun 19. Lab Anim (NY). 2019. PMID: 31217565 Free PMC article. Review.
-
A Region of UNC-89 (Obscurin) Lying between Two Protein Kinase Domains Is a Highly Elastic Spring Required for Proper Sarcomere Organization.J Mol Biol. 2020 Aug 7;432(17):4799-4814. doi: 10.1016/j.jmb.2020.06.024. Epub 2020 Jul 6. J Mol Biol. 2020. PMID: 32645312 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

