A Laser-Guided Spinal Cord Displacement Injury in Adult Mice
- PMID: 29893166
- PMCID: PMC6352504
- DOI: 10.1089/neu.2018.5756
A Laser-Guided Spinal Cord Displacement Injury in Adult Mice
Abstract
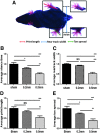
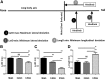
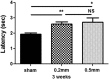
Mouse models are unique for studying molecular mechanisms of neurotrauma because of the availability of various genetic modified mouse lines. For spinal cord injury (SCI) research, producing an accurate injury is essential, but it is challenging because of the small size of the mouse cord and the inconsistency of injury production. The Louisville Injury System Apparatus (LISA) impactor has been shown to produce precise contusive SCI in adult rats. Here, we examined whether the LISA impactor could be used to create accurate and graded contusive SCIs in mice. Adult C57BL/6 mice received a T10 laminectomy followed by 0.2, 0.5, and 0.8 mm displacement injuries, guided by a laser, from the dorsal surface of the spinal cord using the LISA impactor. Basso Mouse Scale (BMS), grid-walking, TreadScan, and Hargreaves analyses were performed for up to 6 weeks post-injury. All mice were euthanized at the 7th week, and the spinal cords were collected for histological analysis. Our results showed that the LISA impactor produced accurate and consistent contusive SCIs corresponding to mild, moderate, and severe injuries to the cord. The degree of injury severities could be readily determined by the BMS locomotor, grid-walking, and TreadScan gait assessments. The cutaneous hyperalgesia threshold was also significantly increased as the injury severity increased. The terminal lesion area and the spared white matter of the injury epicenter were strongly correlated with the injury severities. We conclude that the LISA device, guided by a laser, can produce reliable graded contusive SCIs in mice, resulting in severity-dependent behavioral and histopathological deficits.
Keywords: SCI; behavioral test; contusion; mouse; tissue displacement.
Conflict of interest statement
Dr. C.B. Shields holds an ownership position in LIS, Inc. The other authors have nothing to disclose.
Figures




References
-
- Center., N.S.C.I.S. (2018). Facts and Figures at a Glance. University of Alabama: Birmingham
-
- Cheriyan T., Ryan D.J., Weinreb J.H., Cheriyan J., Paul J.C., Lafage V., Kirsch T., and Errico T.J. (2014). Spinal cord injury models: a review. Spinal Cord 52, 588–595 - PubMed
-
- Young W. (2002). Spinal cord contusion models. Prog. Brain Res. 137, 231–255 - PubMed
-
- Allen R.A. (1911). Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. J.A.M.A. 57, 878–880
-
- Gruner J.A. (1992). A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 9, 123–128 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

