Toward Point-of-Care Drug Quality Assurance in Developing Countries: Comparison of Liquid Chromatography and Infrared Spectroscopy Quantitation of a Small-Scale Random Sample of Amoxicillin
- PMID: 29893196
- PMCID: PMC6090331
- DOI: 10.4269/ajtmh.17-0779
Toward Point-of-Care Drug Quality Assurance in Developing Countries: Comparison of Liquid Chromatography and Infrared Spectroscopy Quantitation of a Small-Scale Random Sample of Amoxicillin
Abstract
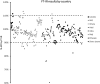
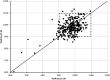
Substandard antibiotics are thought to be a major threat to public health in developing countries and a cause of antimicrobial resistance. However, assessing quality outside of a laboratory setting, using simple equipment, is challenging. The aim of this study was to validate the use of a portable Fourier transform infrared (FT-IR) spectrometer for the identification of substandard antibiotics. Results are presented for amoxicillin packages from Haiti, Ghana, Sierra Leone, Democratic Republic of Congo, India, Papua New Guinea, and Ethiopia collected over the course of 6 months in 2017, including two field trips with the FT-IR to Ghana and Sierra Leone. Canadian samples were used as a control. Regarding drug quality, of 290 individual capsules of amoxicillin analyzed, 13 were found to be substandard with total active pharmaceutical ingredients (API) lying outside the acceptable range of 90-110%. Of these 13, four were below 80% API. The FT-IR reliably identified these outliers and was found to yield results in good agreement with the established pharmacopeia liquid chromatography protocol. We conclude that the portable FT-IR may be suitable to intercept substandard antibiotics in developing countries where more sophisticated techniques are not readily available.
Figures


References
-
- Hollein L, Kaale E, Mwalwisi YH, Schulze MH, Holzgrabe U, 2016. Routine quality control of medicines in developing countries: analytical challenges, regulatory infrastructures and the prevalence of counterfeit medicines in Tanzania. TrAC-Trends Anal Chem 76: 60–70.
-
- WHO , 2012. Substandard/Spurious/Falsely-Labelled/Falsified/Counterfeit Medical Products. Report A/MSM/1/INF/1. Geneva, Switzerland: World Health Organization.
-
- Interpol , 2014. 2004–2014—Ten years of Combating Pharmaceutical Crime: Review and Prospects, Dublin, Ireland 19–20 November 2014. INTERPOL Background Note. Lyon, France: INTERPOL.
-
- Nickerson JW, Attaran A, Westerberg BD, Curtis S, Overton S, Mayer PM, 2016. Fatal bacterial meningitis possibly associated with substandard ceftriaxone—Uganda, 2013. MMWR Morb Mortal Wkly Rep 64: 1375–1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

