Mechanism of neurotransmitter release coming into focus
- PMID: 29893445
- PMCID: PMC6153415
- DOI: 10.1002/pro.3445
Mechanism of neurotransmitter release coming into focus
Abstract
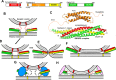
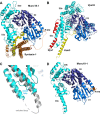
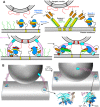
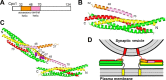
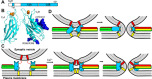
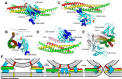
Research for three decades and major recent advances have provided crucial insights into how neurotransmitters are released by Ca2+ -triggered synaptic vesicle exocytosis, leading to reconstitution of basic steps that underlie Ca2+ -dependent membrane fusion and yielding a model that assigns defined functions for central components of the release machinery. The soluble N-ethyl maleimide sensitive factor attachment protein receptors (SNAREs) syntaxin-1, SNAP-25, and synaptobrevin-2 form a tight SNARE complex that brings the vesicle and plasma membranes together and is key for membrane fusion. N-ethyl maleimide sensitive factor (NSF) and soluble NSF attachment proteins (SNAPs) disassemble the SNARE complex to recycle the SNAREs for another round of fusion. Munc18-1 and Munc13-1 orchestrate SNARE complex formation in an NSF-SNAP-resistant manner by a mechanism whereby Munc18-1 binds to synaptobrevin and to a self-inhibited "closed" conformation of syntaxin-1, thus forming a template to assemble the SNARE complex, and Munc13-1 facilitates assembly by bridging the vesicle and plasma membranes and catalyzing opening of syntaxin-1. Synaptotagmin-1 functions as the major Ca2+ sensor that triggers release by binding to membrane phospholipids and to the SNAREs, in a tight interplay with complexins that accelerates membrane fusion. Many of these proteins act as both inhibitors and activators of exocytosis, which is critical for the exquisite regulation of neurotransmitter release. It is still unclear how the actions of these various proteins and multiple other components that control release are integrated and, in particular, how they induce membrane fusion, but it can be expected that these fundamental questions can be answered in the near future, building on the extensive knowledge already available.
Keywords: Munc13; Munc18; NSF; SNAPs; SNAREs; complexin; neurotransmitter release; synaptic vesicle fusion; synaptotagmin.
© 2018 The Protein Society.
Figures

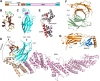
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

