Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer
- PMID: 29893810
- PMCID: PMC6148973
- DOI: 10.1093/carcin/bgy078
Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer
Abstract
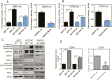
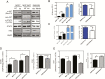
The NF2 gene encodes the tumor and metastasis suppressor protein Merlin. Merlin exerts its tumor suppressive role by inhibiting proliferation and inducing contact-growth inhibition and apoptosis. In the current investigation, we determined that loss of Merlin in breast cancer tissues is concordant with the loss of the inhibitory SMAD, SMAD7, of the TGF-β pathway. This was reflected as dysregulated activation of TGF-β signaling that co-operatively engaged with effectors of the Hippo pathway (YAP/TAZ/TEAD). As a consequence, the loss of Merlin in breast cancer resulted in a significant metabolic and bioenergetic adaptation of cells characterized by increased aerobic glycolysis and decreased oxygen consumption. Mechanistically, we determined that the co-operative activity of the Hippo and TGF-β transcription effectors caused upregulation of the long non-coding RNA Urothelial Cancer-Associated 1 (UCA1) that disengaged Merlin's check on STAT3 activity. The consequent upregulation of Hexokinase 2 (HK2) enabled a metabolic shift towards aerobic glycolysis. In fact, Merlin deficiency engendered cellular dependence on this metabolic adaptation, endorsing a critical role for Merlin in regulating cellular metabolism. This is the first report of Merlin functioning as a molecular restraint on cellular metabolism. Thus, breast cancer patients whose tumors demonstrate concordant loss of Merlin and SMAD7 may benefit from an approach of incorporating STAT3 inhibitors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

