Gyrase containing a single C-terminal domain catalyzes negative supercoiling of DNA by decreasing the linking number in steps of two
- PMID: 29893908
- PMCID: PMC6061840
- DOI: 10.1093/nar/gky470
Gyrase containing a single C-terminal domain catalyzes negative supercoiling of DNA by decreasing the linking number in steps of two
Abstract
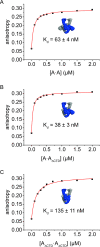
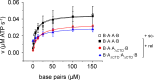
The topological state of DNA in vivo is regulated by topoisomerases. Gyrase is a bacterial topoisomerase that introduces negative supercoils into DNA at the expense of ATP hydrolysis. According to the strand-passage mechanism, a double-strand of the DNA substrate is cleaved, and a second double-stranded segment is passed through the gap, converting a positive DNA node into a negative node. The correct orientation of these DNA segments for strand passage is achieved by wrapping of the DNA around gyrase, which involves the C-terminal domains (CTDs) of both GyrA subunits in the A2B2 heterotetramer. Gyrase lacking both CTDs cannot introduce negative supercoils into DNA. Here, we analyze the requirements for the two CTDs in individual steps in the supercoiling reaction. Gyrase that contains a single CTD binds, distorts, and cleaves DNA similarly to wildtype gyrase. It also shows wildtype-like DNA-dependent ATPase activity, and undergoes DNA-induced movement of the CTD as well as N-gate narrowing. Most importantly, the enzyme still introduces negative supercoils into DNA in an ATP-dependent reaction, with a velocity similar to wildtype gyrase, and decreases the linking number of the DNA in steps of two. One CTD is thus sufficient to support DNA supercoiling.
Figures






References
-
- Brown P.O., Cozzarelli N.R.. A sign inversion mechanism for enzymatic supercoiling of DNA. Science. 1979; 206:1081–1083. - PubMed
-
- Goto T., Wang J.C.. Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J. Biol. Chem. 1982; 257:5866–5872. - PubMed
-
- Wang J.C. Moving one DNA double helix through another by a type II DNA topoisomerase: the story of a simple molecular machine. Q. Rev. Biophys. 1998; 31:107–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

