Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana
- PMID: 29894383
- PMCID: PMC7255068
- DOI: 10.1097/QAD.0000000000001920
Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana
Abstract
: There are limited data on the effectiveness of dolutegravir (DTG)-based combination antiretroviral therapy (ART) in real-life settings in southern Africa where HIV-1 subtype C predominates. We report a patient infected with HIV-1 subtype C on DTG-based ART previously exposed to raltegravir who developed multidrug resistance mutations to four antiretroviral classes. There is need for drug resistance monitoring and clinical vigilance to ensure effectiveness of HIV treatment programs even in the era of DTG-based ART.
Conflict of interest statement
Conflicts of interest
There are no conflicts of interest.
Figures
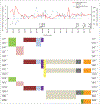
References
-
- UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. http://www.unaid-s.org/sites/default/files/media_asset/90-90-90_en.pdf. [Accessed 3 February 2018]
-
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316:171–181. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services; 2006. http://www.aidsinfo.nih.gov/ContentFiles/Adult. [Accessed 18 January 2018]
-
- EACS guidelines version 9.0; October 2017. http://www.eacsociety.org/files/guidelines_9.0-english.pdf. [Accessed 3 February 2018].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

