Advances in Diagnostic and Intraoperative Molecular Imaging of Pancreatic Cancer
- PMID: 29894417
- PMCID: PMC6003672
- DOI: 10.1097/MPA.0000000000001075
Advances in Diagnostic and Intraoperative Molecular Imaging of Pancreatic Cancer
Abstract
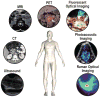
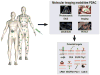
Pancreatic ductal adenocarcinoma (PDAC) has a dismal prognosis. To improve outcomes, there is a critical need for improved tools for detection, accurate staging, and resectability assessment. This could improve patient stratification for the most optimal primary treatment modality. Molecular imaging, used in combination with tumor-specific imaging agents, can improve established imaging methods for PDAC. These novel, tumor-specific imaging agents developed to target specific biomarkers have the potential to specifically differentiate between malignant and benign diseases, such as pancreatitis. When these agents are coupled to various types of labels, this type of molecular imaging can provide integrated diagnostic, noninvasive imaging of PDAC as well as image-guided pancreatic surgery. This review provides a detailed overview of the current clinical imaging applications, upcoming molecular imaging strategies for PDAC, and potential targets for imaging, with an emphasis on intraoperative imaging applications.
Conflict of interest statement
Figures




References
-
- Jang JY, Kang MJ, Heo JS, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259:656–664. - PubMed
-
- Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–20. - PubMed
-
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

