Artemether-lumefantrine dosing for malaria treatment in young children and pregnant women: A pharmacokinetic-pharmacodynamic meta-analysis
- PMID: 29894518
- PMCID: PMC5997317
- DOI: 10.1371/journal.pmed.1002579
Artemether-lumefantrine dosing for malaria treatment in young children and pregnant women: A pharmacokinetic-pharmacodynamic meta-analysis
Abstract
Background: The fixed dose combination of artemether-lumefantrine (AL) is the most widely used treatment for uncomplicated Plasmodium falciparum malaria. Relatively lower cure rates and lumefantrine levels have been reported in young children and in pregnant women during their second and third trimester. The aim of this study was to investigate the pharmacokinetic and pharmacodynamic properties of lumefantrine and the pharmacokinetic properties of its metabolite, desbutyl-lumefantrine, in order to inform optimal dosing regimens in all patient populations.
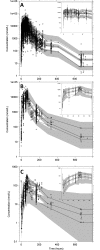
Methods and findings: A search in PubMed, Embase, ClinicalTrials.gov, Google Scholar, conference proceedings, and the WorldWide Antimalarial Resistance Network (WWARN) pharmacology database identified 31 relevant clinical studies published between 1 January 1990 and 31 December 2012, with 4,546 patients in whom lumefantrine concentrations were measured. Under the auspices of WWARN, relevant individual concentration-time data, clinical covariates, and outcome data from 4,122 patients were made available and pooled for the meta-analysis. The developed lumefantrine population pharmacokinetic model was used for dose optimisation through in silico simulations. Venous plasma lumefantrine concentrations 7 days after starting standard AL treatment were 24.2% and 13.4% lower in children weighing <15 kg and 15-25 kg, respectively, and 20.2% lower in pregnant women compared with non-pregnant adults. Lumefantrine exposure decreased with increasing pre-treatment parasitaemia, and the dose limitation on absorption of lumefantrine was substantial. Simulations using the lumefantrine pharmacokinetic model suggest that, in young children and pregnant women beyond the first trimester, lengthening the dose regimen (twice daily for 5 days) and, to a lesser extent, intensifying the frequency of dosing (3 times daily for 3 days) would be more efficacious than using higher individual doses in the current standard treatment regimen (twice daily for 3 days). The model was developed using venous plasma data from patients receiving intact tablets with fat, and evaluations of alternative dosing regimens were consequently only representative for venous plasma after administration of intact tablets with fat. The absence of artemether-dihydroartemisinin data limited the prediction of parasite killing rates and recrudescent infections. Thus, the suggested optimised dosing schedule was based on the pharmacokinetic endpoint of lumefantrine plasma exposure at day 7.
Conclusions: Our findings suggest that revised AL dosing regimens for young children and pregnant women would improve drug exposure but would require longer or more complex schedules. These dosing regimens should be evaluated in prospective clinical studies to determine whether they would improve cure rates, demonstrate adequate safety, and thereby prolong the useful therapeutic life of this valuable antimalarial treatment.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: KIB and NJW are members of the WHO Technical Expert Group (TEG) on Malaria Chemotherapy. KIB is also a member of the WHO TEG on Drug Resistance and Containment. KIB, NJW, JT and SP are members of the WHO Malaria Chemotherapy sub-group on dosage recommendations. GL, KH, FE and RB are employees of Novartis, the manufacturer of the drug that is the subject of this publication. EAA and NJW are members of the Editorial Board of PLOS Medicine. None of the authors declare any other conflict of interest.
Figures



References
-
- World Health Organization. World malaria report 2016 Geneva: World Health Organization; 2016.
-
- World Health Organization. Guidelines for the treatment of malaria Geneva: World Health Organization; 2015.
-
- World Health Organization. World malaria report 2014 Geneva: World Health Organization; 2014.
-
- McGready R, Stepniewska K, Lindegardh N, Ashley EA, La Y, Singhasivanon P, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62(12):1021–31. doi: 10.1007/s00228-006-0199-7 - DOI - PubMed
-
- Kloprogge F, Piola P, Dhorda M, Muwanga S, Turyakira E, Apinan S, et al. Population pharmacokinetics of lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. CPT Pharmacometrics Syst Pharmacol. 2013;2:e83 doi: 10.1038/psp.2013.59 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

