Galectin-3 and N-acetylglucosamine promote myogenesis and improve skeletal muscle function in the mdx model of Duchenne muscular dystrophy
- PMID: 29894670
- PMCID: PMC6219824
- DOI: 10.1096/fj.201701151RRR
Galectin-3 and N-acetylglucosamine promote myogenesis and improve skeletal muscle function in the mdx model of Duchenne muscular dystrophy
Abstract
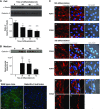
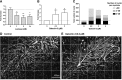
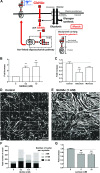
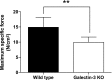
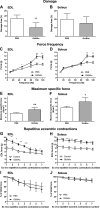
The muscle membrane, sarcolemma, must be firmly attached to the basal lamina. The failure of proper attachment results in muscle injury, which is the underlying cause of Duchenne muscular dystrophy (DMD), in which mutations in the dystrophin gene disrupts the firm adhesion. In patients with DMD, even moderate contraction causes damage, leading to progressive muscle degeneration. The damaged muscles are repaired through myogenesis. Consequently, myogenesis is highly active in patients with DMD, and the repeated activation of myogenesis leads to the exhaustion of the myogenic stem cells. Therefore, approaches to reducing the risk of the exhaustion are to develop a treatment that strengthens the interaction between the sarcolemma and the basal lamina and increases the efficiency of the myogenesis. Galectin-3 is an oligosaccharide-binding protein and is known to be involved in cell-cell interactions and cell-matrix interactions. Galectin-3 is expressed in myoblasts and skeletal muscle, although its function in muscle remains elusive. In this study, we found evidence that galectin-3 and the monosaccharide N-acetylglucosamine, which increases the synthesis of binding partners (oligosaccharides) of galectin-3, promote myogenesis in vitro. Moreover, in the mdx mouse model of DMD, treatment with N-acetylglucosamine increased muscle-force production. The results suggest that treatment with N-acetylglucosamine might mitigate the burden of DMD.-Rancourt, A., Dufresne, S. S., St-Pierre, G., Lévesque, J.-C., Nakamura, H., Kikuchi, Y., Satoh, M. S., Frenette, J., Sato, S. Galectin-3 and N-acetylglucosamine promote myogenesis and improve skeletal muscle function in the mdx model of Duchenne muscular dystrophy.
Keywords: glycobiology; lectins; monosaccharide.
Conflict of interest statement
The authors acknowledge the Bioimaging Platform at the Centre de Recherche Centre Hospitalier Universitaire (CHU) de Quebec. The authors thank the Consortium for Functional Glycomics for galectin-3 knockout mice. The mAb directed against MHC was obtained from the Developmental Studies Hybridoma Bank, created by the U.S. National Institutes of Health, Institute of Child Health and Human Development, and maintained at the Department of Biology, University of Iowa (Iowa City, IA, USA). The authors declare no conflicts of interest.
Figures
References
-
- Cummings R. D., Liu F.-T., Vasta G. R. (2017) Galectins. In: Essentials of Glycobiology [Internet], 3rd ed. (Varki, A., Esko, J. D., Stanley, P., Hart, G. W., Aebi, M., Darvill, A.G ., Kinoshita, T., Packer, N. H., Prestegard, J. H., Schnaar, R. L., Seeberger, P. H., Cummings, R. D., Liu, F. T., Vasta, G. R., eds.), Chapter 36, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA
-
- Sato S., St-Pierre C., Bhaumik P., Nieminen J. (2009) Galectins in innate immunity: dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 230, 172–187 - PubMed
-
- Rabinovich G. A., Toscano M. A. (2009) Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 - PubMed
-
- Hirabayashi J., Kasai K. (1993) The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3, 297–304 - PubMed
-
- Sato S., Hughes R. C. (1994) Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 269, 4424–4430 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

