Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs
- PMID: 29894913
- PMCID: PMC6082159
- DOI: 10.1016/j.biomaterials.2018.04.049
Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs
Abstract
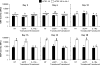
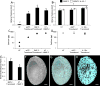
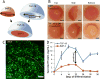
Cartilage-derived matrix (CDM) has emerged as a promising scaffold material for tissue engineering of cartilage and bone due to its native chondroinductive capacity and its ability to support endochondral ossification. Because it consists of native tissue, CDM can undergo cellular remodeling, which can promote integration with host tissue and enables it to be degraded and replaced by neotissue over time. However, enzymatic degradation of decellularized tissues can occur unpredictably and may not allow sufficient time for mechanically competent tissue to form, especially in the harsh inflammatory environment of a diseased joint. The goal of the current study was to engineer cartilage and bone constructs with the ability to inhibit aberrant inflammatory processes caused by the cytokine interleukin-1 (IL-1), through scaffold-mediated delivery of lentiviral particles containing a doxycycline-inducible IL-1 receptor antagonist (IL-1Ra) transgene on anatomically-shaped CDM constructs. Additionally, scaffold-mediated lentiviral gene delivery was used to facilitate spatial organization of simultaneous chondrogenic and osteogenic differentiation via site-specific transduction of a single mesenchymal stem cell (MSC) population to overexpress either chondrogenic, transforming growth factor-beta 3 (TGF-β3), or osteogenic, bone morphogenetic protein-2 (BMP-2), transgenes. Controlled induction of IL-1Ra expression protected CDM hemispheres from inflammation-mediated degradation, and supported robust bone and cartilage tissue formation even in the presence of IL-1. In the absence of inflammatory stimuli, controlled cellular remodeling was exploited as a mechanism for fusing concentric CDM hemispheres overexpressing BMP-2 and TGF-β3 into a single bi-layered osteochondral construct. Our findings demonstrate that site-specific delivery of inducible and tunable transgenes confers spatial and temporal control over both CDM scaffold remodeling and neotissue composition. Furthermore, these constructs provide a microphysiological in vitro joint organoid model with site-specific, tunable, and inducible protein delivery systems for examining the spatiotemporal response to pro-anabolic and/or inflammatory signaling across the osteochondral interface.
Keywords: Cartilage repair; Gene activated matrix; Gene therapy; Immunoengineering; Osteoarthritis; Regenerative medicine.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest: F.G. is a paid employee of Cytex Therapeutics.
Figures

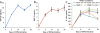




Similar articles
-
Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds.Biomaterials. 2016 Jun;91:57-72. doi: 10.1016/j.biomaterials.2016.03.012. Epub 2016 Mar 9. Biomaterials. 2016. PMID: 26999455 Free PMC article.
-
Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing.Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):E4513-22. doi: 10.1073/pnas.1601639113. Epub 2016 Jul 18. Proc Natl Acad Sci U S A. 2016. PMID: 27432980 Free PMC article.
-
Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration.Acta Biomater. 2017 Nov;63:210-226. doi: 10.1016/j.actbio.2017.09.008. Epub 2017 Sep 9. Acta Biomater. 2017. PMID: 28899816
-
Therapeutic "Tool" in Reconstruction and Regeneration of Tissue Engineering for Osteochondral Repair.Appl Biochem Biotechnol. 2020 Jun;191(2):785-809. doi: 10.1007/s12010-019-03214-8. Epub 2019 Dec 21. Appl Biochem Biotechnol. 2020. PMID: 31863349 Review.
-
Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine.Stem Cell Rev Rep. 2023 Aug;19(6):1615-1634. doi: 10.1007/s12015-023-10545-x. Epub 2023 Apr 19. Stem Cell Rev Rep. 2023. PMID: 37074547 Review.
Cited by
-
rAAV-Mediated Overexpression of SOX9 and TGF-β via Carbon Dot-Guided Vector Delivery Enhances the Biological Activities in Human Bone Marrow-Derived Mesenchymal Stromal Cells.Nanomaterials (Basel). 2020 Apr 28;10(5):855. doi: 10.3390/nano10050855. Nanomaterials (Basel). 2020. PMID: 32354138 Free PMC article.
-
Fabricating the cartilage: recent achievements.Cytotechnology. 2023 Aug;75(4):269-292. doi: 10.1007/s10616-023-00582-2. Epub 2023 May 26. Cytotechnology. 2023. PMID: 37389132 Free PMC article. Review.
-
Current Trends in Viral Gene Therapy for Human Orthopaedic Regenerative Medicine.Tissue Eng Regen Med. 2019 Feb 21;16(4):345-355. doi: 10.1007/s13770-019-00179-x. eCollection 2019 Aug. Tissue Eng Regen Med. 2019. PMID: 31413939 Free PMC article. Review.
-
Implication of Mesenchymal Stem Cells and Their Derivates for Osteochondral Regeneration.Int J Mol Sci. 2022 Feb 24;23(5):2490. doi: 10.3390/ijms23052490. Int J Mol Sci. 2022. PMID: 35269633 Free PMC article. Review.
-
Administration of mRNA-Nanomedicine-Augmented Calvarial Defect Healing via Endochondral Ossification.Pharmaceutics. 2023 Jul 17;15(7):1965. doi: 10.3390/pharmaceutics15071965. Pharmaceutics. 2023. PMID: 37514151 Free PMC article.
References
-
- Jiang Y, Lin H, Tuan RS. Overview: State of the Art and Future Prospectives for Cartilage Repair. In: Grässel S, Aszódi A, editors. Cartilage: Volume 3: Repair Strategies and Regeneration. Springer International Publishing; Cham: 2017. pp. 1–34.
-
- Lopa S, Madry H. Bioinspired scaffolds for osteochondral regeneration. Tissue Eng Part A. 2014;20(15–16):2052–76. - PubMed
-
- Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32(21):4793–805. - PubMed
-
- Han F, Zhou F, Yang X, Zhao J, Zhao Y, Yuan X. A pilot study of conically graded chitosan–gelatin hydrogel/PLGA scaffold with dual‐delivery of TGF‐β1 and BMP‐2 for regeneration of cartilage–bone interface. J Biomed Mater Res B Appl Biomater. 2015;103(7):1344–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

