Stereocontrolled Synthesis of α-Xylofuranosides Using a Conformationally Restricted Donor
- PMID: 29895148
- PMCID: PMC6079929
- DOI: 10.1021/acs.joc.8b00410
Stereocontrolled Synthesis of α-Xylofuranosides Using a Conformationally Restricted Donor
Abstract
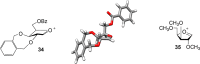
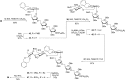
A number of biologically relevant glycoconjugates possess 1,2- cis-furanosidic linkages, a class of glycosidic bond that remains challenging to introduce with high stereoselectivity. In this paper, we report an approach to one family of such linkages, α-xylofuranosides, via the use of thioglycoside donors possessing a conformationally restricting xylylene protecting group. The method was shown to provide the desired targets in good to excellent yield and stereoselectivity. Computational investigations support the proposal that the protecting group locks the electrophilic intermediate in these reactions into a conformation that leads to the high selectivity. The power of the methodology was demonstrated through the synthesis of a complex hexasaccharide motif from lipoarabinomannan, an immunomodulatory polysaccharide from mycobacteria.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



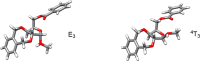


Similar articles
-
Stereoselective Synthesis of α-3-Deoxy-D-manno-oct-2-ulosonic Acid (α-Kdo) Glycosides Using 5,7-O-Di-tert-butylsilylene-Protected Kdo Ethyl Thioglycoside Donors.Angew Chem Int Ed Engl. 2015 Sep 7;54(37):10894-8. doi: 10.1002/anie.201505176. Epub 2015 Jul 23. Angew Chem Int Ed Engl. 2015. PMID: 26205626
-
2,3-Anhydro sugars in glycoside bond synthesis. Highly stereoselective syntheses of oligosaccharides containing alpha- and beta-arabinofuranosyl linkages.J Am Chem Soc. 2003 Apr 9;125(14):4155-65. doi: 10.1021/ja029302m. J Am Chem Soc. 2003. PMID: 12670238
-
Conformationally restricted 3,5-O-(di-tert-butylsilylene)-D-galactofuranosyl thioglycoside donor for 1,2-cis α-D-galactofuranosylation.Carbohydr Res. 2014 Oct 9;397:7-17. doi: 10.1016/j.carres.2014.07.024. Epub 2014 Aug 7. Carbohydr Res. 2014. PMID: 25168009
-
Chiral auxiliaries: Usefullness in stereoselective glycosylation reactions and their synthetic applications.Carbohydr Res. 2020 Sep;495:108045. doi: 10.1016/j.carres.2020.108045. Epub 2020 Jul 7. Carbohydr Res. 2020. PMID: 32679340 Review.
-
Assembly of naturally occurring glycosides, evolved tactics, and glycosylation methods.Acc Chem Res. 2012 Aug 21;45(8):1227-36. doi: 10.1021/ar200296m. Epub 2012 Apr 11. Acc Chem Res. 2012. PMID: 22493991 Review.
Cited by
-
Stereospecific Furanosylations Catalyzed by Bis-thiourea Hydrogen-Bond Donors.J Am Chem Soc. 2020 Feb 26;142(8):4061-4069. doi: 10.1021/jacs.0c00335. Epub 2020 Feb 14. J Am Chem Soc. 2020. PMID: 32013410 Free PMC article.
-
Furanosyl Oxocarbenium Ion Conformational Energy Landscape Maps as a Tool to Study the Glycosylation Stereoselectivity of 2-Azidofuranoses, 2-Fluorofuranoses and Methyl Furanosyl Uronates.Chemistry. 2019 May 23;25(29):7149-7157. doi: 10.1002/chem.201900651. Epub 2019 Apr 17. Chemistry. 2019. PMID: 30882938 Free PMC article.
-
ZnI2-Mediated cis-Glycosylations of Various Constrained Glycosyl Donors: Recent Advances in cis-Selective Glycosylations.Molecules. 2024 Oct 4;29(19):4710. doi: 10.3390/molecules29194710. Molecules. 2024. PMID: 39407638 Free PMC article. Review.
-
B(C6F5)3-Catalyzed Stereoselective 1,2-cis Arabinofuranosylation with a Conformationally Constrained Donor.ACS Omega. 2024 Mar 4;9(10):11969-11975. doi: 10.1021/acsomega.3c09761. eCollection 2024 Mar 12. ACS Omega. 2024. PMID: 38497025 Free PMC article.
-
An SN2-Type Strategy toward 1,2-cis-Furanosides.CCS Chem. 2022 Dec;4(12):3677-3685. doi: 10.31635/ccschem.022.202202175. Epub 2022 Dec 7. CCS Chem. 2022. PMID: 38186678 Free PMC article.
References
-
- Marino C.; Gallo-Rodriguez C.; de Lederkremer R. M.. Galactofuranosyl-Containing Glycans: Occurrence, Synthesis and Biochemistry. In Glycans: Biochemistry, Characterization and Applications, first ed.; Mora-Montes H. M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, 2012; pp 207–268.
-
- Imamura A.; Lowary T. L. Chemical Synthesis of Furanose Glycosides. Trends Glycosci. Glycotechnol. 2011, 23, 134–152. 10.4052/tigg.23.134. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

