A randomized, observer-blinded, equivalence trial comparing two variations of Euvichol®, a bivalent killed whole-cell oral cholera vaccine, in healthy adults and children in the Philippines
- PMID: 29895500
- PMCID: PMC6026293
- DOI: 10.1016/j.vaccine.2018.05.102
A randomized, observer-blinded, equivalence trial comparing two variations of Euvichol®, a bivalent killed whole-cell oral cholera vaccine, in healthy adults and children in the Philippines
Abstract
Background: To contribute to the global demand for oral cholera vaccine (OCV), the production of Euvichol® was scaled up with elimination of thimerosal. To demonstrate the equivalence of the variations, a study was carried out in the Philippines.
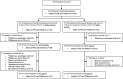
Methods: Healthy male and female adults and children in Manila were randomized to receive two doses of Euvichol® two weeks apart from either the 100L (Comparator) or the 600L (Test) variation. Primary and secondary immunogenicity endpoints were respectively geometric mean titer (GMT) of vibriocidal antibodies (two weeks post second dose) and seroconversion rate (two weeks after each dose) against O1 Inaba, Ogawa, and O139 serogroups. The GMT of vibriocidal antibodies against O1 Inaba, Ogawa, and O139 two weeks post first dose was also measured. To show the equivalence of two variations of Euvichol®, the ratio of GMT and the difference of seroconversion rate between Test and Comparator vaccines were tested with equivalence margin of [0.5, 2.0] for GMT ratio and of 15% for seroconversion rate, respectively. Safety assessment included solicited reactogenicity within 6 days after each dose and unsolicited and serious adverse events.
Results: A total of 442 participants were enrolled. For the overall population, equivalence between Test and Comparator was demonstrated for vibriocidal antibody response against O1 Inaba and Ogawa serotypes and O139 serogroup in both modified intention-to-treat (mITT) and per protocol analysis, since the 95% confidence intervals (CI) of GMT to any serotypes were within the lower and upper boundary [0.5, 2.0]. Seroconversion rates after two doses also showed equivalence for O1 Inaba, Ogawa, and O139. The vaccine was safe and well tolerated, similarly between the two groups.
Conclusion: The study results support the equivalence of the 600L Euvichol® to the 100L formulation in healthy children and adults. The 600L Euvichol® is safe and immunogenic in adults and children. ClinicalTrials.gov registration number: NCT02502331.
Keywords: Cholera; Equivalence; Euvichol; Oral cholera vaccine; The Philippines.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Weekly epidemiological record Cholera vaccines: WHO position paper, 26 MARCH 2010, 85th YEAR, 85e ANNÉE No. 13, 2010;85:117–12.
-
- Weekly epidemiological record. Cholera vaccines: WHO position paper – August 2017, 92th YEAR - No 34, 2017;92:477–500.
-
- World Health Assembly. Resolution 64.18. Cholera: mechanism for control and prevention. Sixty-fourth World Health Assembly, 17 March 2011. Geneva, Switzerland: World Health Organization, 2011.
-
- World Health Organization. WHO technical working group on the creation of an oral cholera vaccine stockpile: meeting report. Geneva SWDPS, 2012.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

