Cerebellar synapse properties and cerebellum-dependent motor and non-motor performance in Dp71-null mice
- PMID: 29895670
- PMCID: PMC6078407
- DOI: 10.1242/dmm.033258
Cerebellar synapse properties and cerebellum-dependent motor and non-motor performance in Dp71-null mice
Abstract
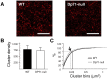
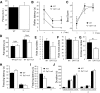
Recent emphasis has been placed on the role that cerebellar dysfunctions could have in the genesis of cognitive deficits in Duchenne muscular dystrophy (DMD). However, relevant genotype-phenotype analyses are missing to define whether cerebellar defects underlie the severe cases of intellectual deficiency that have been associated with genetic loss of the smallest product of the dmd gene, the Dp71 dystrophin. To determine for the first time whether Dp71 loss could affect cerebellar physiology and functions, we have used patch-clamp electrophysiological recordings in acute cerebellar slices and a cerebellum-dependent behavioral test battery addressing cerebellum-dependent motor and non-motor functions in Dp71-null transgenic mice. We found that Dp71 deficiency selectively enhances excitatory transmission at glutamatergic synapses formed by climbing fibers (CFs) on Purkinje neurons, but not at those formed by parallel fibers. Altered basal neurotransmission at CFs was associated with impairments in synaptic plasticity and clustering of the scaffolding postsynaptic density protein PSD-95. At the behavioral level, Dp71-null mice showed some improvements in motor coordination and were unimpaired for muscle force, static and dynamic equilibrium, motivation in high-motor demand and synchronization learning. Dp71-null mice displayed altered strategies in goal-oriented navigation tasks, however, suggesting a deficit in the cerebellum-dependent processing of the procedural components of spatial learning, which could contribute to the visuospatial deficits identified in this model. In all, the observed deficits suggest that Dp71 loss alters cerebellar synapse function and cerebellum-dependent navigation strategies without being detrimental for motor functions.
Keywords: Cerebellum; Cognitive deficit; Dp71; Dystrophin; Glutamatergic transmission; Motor coordination; Purkinje neuron.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Anderson J. L., Head S. I. and Morley J. W. (2003). Altered inhibitory input to Purkinje cells of dystrophin-deficient mice. Brain Res. 982, 280-283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

