Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection
- PMID: 29895826
- PMCID: PMC6278832
- DOI: 10.1038/s41577-018-0025-3
Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection
Abstract
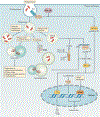
Mycobacterium tuberculosis is a leading cause of mortality worldwide and establishes a long-lived latent infection in a substantial proportion of the human population. Multiple lines of evidence suggest that some individuals are resistant to latent M. tuberculosis infection despite long-term and intense exposure, and we term these individuals 'resisters'. In this Review, we discuss the epidemiological and genetic data that support the existence of resisters and propose criteria to optimally define and characterize the resister phenotype. We review recent insights into the immune mechanisms of M. tuberculosis clearance, including responses mediated by macrophages, T cells and B cells. Understanding the cellular mechanisms that underlie resistance to M. tuberculosis infection may reveal immune correlates of protection that could be utilized for improved diagnostics, vaccine development and novel host-directed therapeutic strategies.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
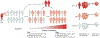


References
-
- Dye C, Scheele S, Dolin P, Pathania V & Raviglione MC Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA 282, 677–686 (1999). - PubMed
-
- World Health Organization. Global Tuberculosis Report 2017. (WHO, 2017).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

