Europe-wide reassessment of Dictyocoela (Microsporidia) infecting native and invasive amphipods (Crustacea): molecular versus ultrastructural traits
- PMID: 29895884
- PMCID: PMC5997659
- DOI: 10.1038/s41598-018-26879-3
Europe-wide reassessment of Dictyocoela (Microsporidia) infecting native and invasive amphipods (Crustacea): molecular versus ultrastructural traits
Abstract
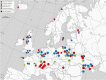
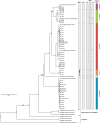
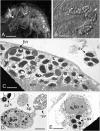
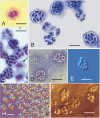
Microsporidia are common parasites infecting animals and protists. They are specifically common pathogens of amphipods (Crustacea, Malacostraca), with Dictyocoela spp. being particularly frequent and highly prevalent, exhibiting a range of phenotypic and ecological effects. Until now, seven species of Dictyocoela were defined, predominantly based on the genetic distance. However, neither the taxonomic status of this provisionally erected genus (based on eight novel sequences and one micrograph of the spore), nor its internal phylogenetic relationships have been clearly revealed. The formal description of the genus and of most of the putative species are still lacking. Here we aimed to fill this gap and performed both ultrastructural and molecular studies (based on SSU, ITS and partial LSU) using different species delimitation methods. As a consensus of these results and following conservative data interpretation, we propose to distinguish five species infecting gammarid hosts, and to keep the names introduced by the authors of the type sequences: Dictyocoela duebenum, D. muelleri, D. berillonum and D. roeselum. We provide full descriptions of these species. Moreover, thanks to our extensive sampling, we extend the known host and geographic range of these Microsporidia.
Conflict of interest statement
The authors declare no competing interests.
Figures
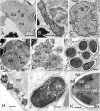
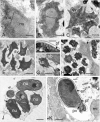
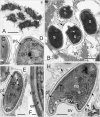
References
-
- Bulnheim HP. Microsporidian infections of amphipods with special reference to host parasite relationships: a review. Mar. Fish. Rev. 1975;37:39–45.
-
- Stentiford, G. D. & Dunn, A. M. Microsporidia in aquatic invertebrates. In Microsporidia: Pathogens of Opportunity (eds Weiss, L. M. & Becnel, J. J.) 579–604 (John Wiley & Sons, Inc., 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

