Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size
- PMID: 29895892
- PMCID: PMC6292748
- DOI: 10.1038/s41380-018-0078-5
Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size
Abstract
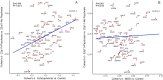
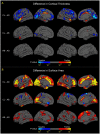
The 22q11.2 deletion (22q11DS) is a common chromosomal microdeletion and a potent risk factor for psychotic illness. Prior studies reported widespread cortical changes in 22q11DS, but were generally underpowered to characterize neuroanatomic abnormalities associated with psychosis in 22q11DS, and/or neuroanatomic effects of variability in deletion size. To address these issues, we developed the ENIGMA (Enhancing Neuro Imaging Genetics Through Meta-Analysis) 22q11.2 Working Group, representing the largest analysis of brain structural alterations in 22q11DS to date. The imaging data were collected from 10 centers worldwide, including 474 subjects with 22q11DS (age = 18.2 ± 8.6; 46.9% female) and 315 typically developing, matched controls (age = 18.0 ± 9.2; 45.9% female). Compared to controls, 22q11DS individuals showed thicker cortical gray matter overall (left/right hemispheres: Cohen's d = 0.61/0.65), but focal thickness reduction in temporal and cingulate cortex. Cortical surface area (SA), however, showed pervasive reductions in 22q11DS (left/right hemispheres: d = -1.01/-1.02). 22q11DS cases vs. controls were classified with 93.8% accuracy based on these neuroanatomic patterns. Comparison of 22q11DS-psychosis to idiopathic schizophrenia (ENIGMA-Schizophrenia Working Group) revealed significant convergence of affected brain regions, particularly in fronto-temporal cortex. Finally, cortical SA was significantly greater in 22q11DS cases with smaller 1.5 Mb deletions, relative to those with typical 3 Mb deletions. We found a robust neuroanatomic signature of 22q11DS, and the first evidence that deletion size impacts brain structure. Psychotic illness in this highly penetrant deletion was associated with similar neuroanatomic abnormalities to idiopathic schizophrenia. These consistent cross-site findings highlight the homogeneity of this single genetic etiology, and support the suitability of 22q11DS as a biological model of schizophrenia.
Conflict of interest statement
DMcD is a member of the Speaker’s Bureau for Natera. The remaining authors declare that they have no conflict of interest.
Figures


References
-
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–8. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 MH087626/MH/NIMH NIH HHS/United States
- T32 MH073526/MH/NIMH NIH HHS/United States
- 100202/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- U54 EB020403/EB/NIBIB NIH HHS/United States
- U01 MH101724/MH/NIMH NIH HHS/United States
- R37 MH085953/MH/NIMH NIH HHS/United States
- U01 MH101719/MH/NIMH NIH HHS/United States
- U54 HD087101/HD/NICHD NIH HHS/United States
- R01 MH085953/MH/NIMH NIH HHS/United States
- P50 HD105354/HD/NICHD NIH HHS/United States
- R01 MH100900/MH/NIMH NIH HHS/United States
- U54 HD086984/HD/NICHD NIH HHS/United States
- MR/N026063/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- K01 MH112774/MH/NIMH NIH HHS/United States
- 102003/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- U01 MH101723/MH/NIMH NIH HHS/United States
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
- U01 MH087636/MH/NIMH NIH HHS/United States
- RC2 MH089983/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

