Repositioning of Omarigliptin as a once-weekly intranasal Anti-parkinsonian Agent
- PMID: 29895906
- PMCID: PMC5997767
- DOI: 10.1038/s41598-018-27395-0
Repositioning of Omarigliptin as a once-weekly intranasal Anti-parkinsonian Agent
Abstract
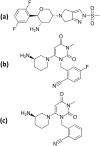
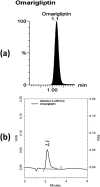
Drug repositioning is a revolution breakthrough of drug discovery that presents outstanding privilege with already safer agents by scanning the existing candidates as therapeutic switching or repurposing for marketed drugs. Sitagliptin, vildagliptin, saxagliptin & linagliptin showed antioxidant and neurorestorative effects in previous studies linked to DPP-4 inhibition. Literature showed that gliptins did not cross the blood brain barrier (BBB) while omarigliptin was the first gliptin that crossed it successfully in the present work. LC-MS/MS determination of once-weekly anti-diabetic DPP-4 inhibitors; omarigliptin & trelagliptin in plasma and brain tissue was employed after 2 h of oral administration to rats. The brain/plasma concentration ratio was used to deduce the penetration power through the BBB. Results showed that only omarigliptin crossed the BBB due to its low molecular weight & lipophilic properties suggesting its repositioning as antiparkinsonian agent. The results of BBB crossing will be of interest for researchers interested in Parkinson's disease. A novel intranasal formulation was developed using sodium lauryl sulphate surfactant to solubilize the lipophilic omarigliptin with penetration enhancing & antimicrobial properties. Intranasal administration showed enhanced brain/plasma ratio by 3.3 folds compared to the oral group accompanied with 2.6 folds increase in brain glucagon-like peptide-1 concentration compared to the control group.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

