Tau-targeting therapies for Alzheimer disease
- PMID: 29895964
- PMCID: PMC6463489
- DOI: 10.1038/s41582-018-0013-z
Tau-targeting therapies for Alzheimer disease
Abstract
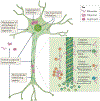
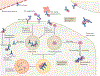
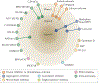
Alzheimer disease (AD) is the most common form of dementia. Pathologically, AD is characterized by amyloid plaques and neurofibrillary tangles in the brain, with associated loss of synapses and neurons, resulting in cognitive deficits and eventually dementia. Amyloid-β (Aβ) peptide and tau protein are the primary components of the plaques and tangles, respectively. In the decades since Aβ and tau were identified, development of therapies for AD has primarily focused on Aβ, but tau has received more attention in recent years, in part because of the failure of various Aβ-targeting treatments in clinical trials. In this article, we review the current status of tau-targeting therapies for AD. Initially, potential anti-tau therapies were based mainly on inhibition of kinases or tau aggregation, or on stabilization of microtubules, but most of these approaches have been discontinued because of toxicity and/or lack of efficacy. Currently, the majority of tau-targeting therapies in clinical trials are immunotherapies, which have shown promise in numerous preclinical studies. Given that tau pathology correlates better with cognitive impairments than do Aβ lesions, targeting of tau is expected to be more effective than Aβ clearance once the clinical symptoms are evident. With future improvements in diagnostics, these two hallmarks of the disease might be targeted prophylactically.
Conflict of interest statement
Competing interests statement
E.M.S. is an inventor on various patents on immunotherapies and related diagnostics that are assigned to New York University. Some of those focusing on the tau protein are licensed to and are being co-developed with H. Lundbeck A/S. E.E.C. declares no competing interests.
Figures
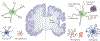
References
-
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509 (2016). - PubMed
-
- Braak H, Thal DR, Ghebremedhin E & Del Tredici K Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

