Activation of Dorsomedial Hypothalamic Neurons Promotes Physical Activity and Decreases Food Intake and Body Weight in Zucker Fatty Rats
- PMID: 29896090
- PMCID: PMC5987017
- DOI: 10.3389/fnmol.2018.00179
Activation of Dorsomedial Hypothalamic Neurons Promotes Physical Activity and Decreases Food Intake and Body Weight in Zucker Fatty Rats
Abstract
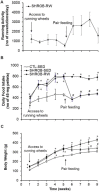
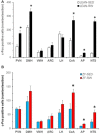
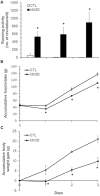
Previous reports have shown that running wheel activity or voluntary exercise prevents hyperphagia and obesity in various animal models of obesity, but such effects seem only minimal in obese animals lacking leptin or leptin receptors. The mechanisms underlying this ineffectiveness remain unclear. Here, we identified the action of neuronal activation in the dorsomedial hypothalamus (DMH) in modulating physical activity, food intake and body weight using leptin receptor mutant obese Zucker (Lepr(fa), ZF) and Koletsky (Lepr(fak), SHROB) rats. Ad lib-fed SHROB rats with locked running wheels became hyperphagic and gained body weight rapidly. These alterations were not ameliorated in ad lib-fed SHROB rats with voluntary access to running wheels, but the body weight of SHROB rats with running wheel access was significantly decreased when they were pair-fed to the amounts consumed by lean controls. Determinations of hypothalamic gene expression revealed that sedentary ad lib-fed SHROB rats had increased expression of neuropeptide Y (Npy) and decreased expression of pro-opiomelanocortin (Pomc) in the arcuate nucleus (ARC). Both ARC Npy and Pomc expression were further altered under running and pair-fed conditions, indicating that both genes are appropriately regulated in response to increased energy demands or alterations caused by running activity and food restriction. Strikingly, c-Fos immunohistochemistry revealed that while voluntary running activity elevated the number of c-Fos positive cells in the DMH (particularly in the ventral and caudal subregions) of intact rats, such activation was not observed in ZF rats. Using adeno-associated virus (AAV)-mediated expression of the designer receptors hM3D(Gq) in the ventral and caudal DMH of ZF rats, we found that chemogenetic stimulation of neurons in these DMH subregions via injection of the designer drug clozapine N-oxide (CNO) significantly increased their running activity and reduced their food intake and body weight. Together, these results demonstrate that activation of ventral and caudal DMH neurons promotes physical activity and decreases food intake and body weight and suggest that intact DMH neural signaling is likely crucial for exercise-induced reductions of food intake and body weight in obese rats lacking leptin receptors.
Keywords: body weight; dorsomedial hypothalamus; food intake; leptin; leptin receptor; obesity; physical activity; running wheel.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

