Increased ATP and ADO Overflow From Sympathetic Nerve Endings and Mesentery Endothelial Cells Plus Reduced Nitric Oxide Are Involved in Diabetic Neurovascular Dysfunction
- PMID: 29896104
- PMCID: PMC5987002
- DOI: 10.3389/fphar.2018.00546
Increased ATP and ADO Overflow From Sympathetic Nerve Endings and Mesentery Endothelial Cells Plus Reduced Nitric Oxide Are Involved in Diabetic Neurovascular Dysfunction
Abstract
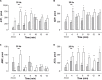
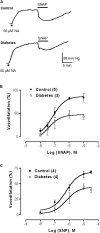
Since the mechanism of human diabetic peripheral neuropathy and vascular disease in type 1 diabetes mellitus remains unknown, we assessed whether sympathetic transmitter overflow is altered by this disease and associated to vascular dysfunction. Diabetes was induced by streptozotocin (STZ)-treatment and compared to vehicle-treated rats. Aliquots of the ex vivo perfused rat arterial mesenteric preparation, denuded of the endothelial layer, were collected to quantify analytically sympathetic nerve co-transmitters overflow secreted by the isolated mesenteries of both groups of rats. Noradrenaline (NA), neuropeptide tyrosine (NPY), and ATP/metabolites were detected before, during, and after electrical field stimulation (EFS, 20 Hz) of the nerve terminals surrounding the mesenteric artery. NA overflow was comparable in both groups; however, basal or EFS-secreted ir-NPY was 26% reduced (p < 0.05) in diabetics. Basal and EFS-evoked ATP and adenosine (ADO) overflow to the arterial mesentery perfusate increased twofold and was longer lasting in diabetics; purine tissue content was 37.8% increased (p < 0.05) in the mesenteries from STZ-treated group of rats. Perfusion of the arterial mesentery vascular territory with 100 μM ATP, 100 nM 2-MeSADP, or 1 μM UTP elicited vasodilator responses of the same magnitude in controls or diabetics, but the increase in luminally accessible NO was 60-70% lower in diabetics (p < 0.05). Moreover, the concentration-response curve elicited by two NO donors was displaced downwards (p < 0.01) in diabetic rats. Parallel studies using primary cultures of endothelial cells from the arterial mesentery vasculature revealed that mechanical stimulation induced a rise in extracellular nucleotides, which in the cells from diabetic rats was larger and longer-lasting when comparing the extracellular release of ATP and ADO values to those of vehicle-treated controls. A 5 min challenge with purinergic agonists elicited a cell media NO rise, which was reduced in the endothelial cells from diabetic rats. Present findings provide neurochemical support for the diabetes-induced neuropathy and show that mesenteric endothelial cells alterations in response to mechanical stimulation are compatible with the endothelial dysfunction related to vascular disease progress.
Keywords: Streptozotocin-induced diabetes; cultured endothelial cells; extracellular adenosine; nitric oxide production; nucleotide release.
Figures



Similar articles
-
Nanomolar clodronate induces adenosine accumulation in the perfused rat mesenteric bed and mesentery-derived endothelial cells.Front Pharmacol. 2023 Jan 20;13:1031223. doi: 10.3389/fphar.2022.1031223. eCollection 2022. Front Pharmacol. 2023. PMID: 36744214 Free PMC article.
-
Reciprocal sympatho-sensory control: functional role of nucleotides and calcitonin gene-related peptide in a peripheral neuroeffector junction.Neuroscience. 2012 Feb 17;203:216-29. doi: 10.1016/j.neuroscience.2011.11.067. Epub 2011 Dec 9. Neuroscience. 2012. PMID: 22178987
-
Presynaptic alpha2-adrenoceptor-mediated modulation of adenosine 5' triphosphate and noradrenaline corelease: differences in canine mesenteric artery and vein.J Auton Pharmacol. 2001 Feb;21(1):47-55. doi: 10.1046/j.1365-2680.2001.00207.x. J Auton Pharmacol. 2001. PMID: 11422578
-
Cotransmission from sympathetic vasoconstrictor neurons: differences in guinea-pig mesenteric artery and vein.Auton Neurosci. 2000 Dec 28;86(1-2):18-29. doi: 10.1016/S1566-0702(00)00203-4. Auton Neurosci. 2000. PMID: 11269921
-
Impaired sensory-motor nerve function in the isolated mesenteric arterial bed of streptozotocin-diabetic and ganglioside-treated streptozotocin-diabetic rats.Br J Pharmacol. 1993 Nov;110(3):1105-11. doi: 10.1111/j.1476-5381.1993.tb13928.x. Br J Pharmacol. 1993. PMID: 8298799 Free PMC article.
Cited by
-
Nanomolar clodronate induces adenosine accumulation in the perfused rat mesenteric bed and mesentery-derived endothelial cells.Front Pharmacol. 2023 Jan 20;13:1031223. doi: 10.3389/fphar.2022.1031223. eCollection 2022. Front Pharmacol. 2023. PMID: 36744214 Free PMC article.
-
Alteration of purinergic signaling in diabetes: Focus on vascular function.J Mol Cell Cardiol. 2020 Mar;140:1-9. doi: 10.1016/j.yjmcc.2020.02.004. Epub 2020 Feb 11. J Mol Cell Cardiol. 2020. PMID: 32057736 Free PMC article. Review.
-
Purinergic Signaling During Hyperglycemia in Vascular Smooth Muscle Cells.Front Endocrinol (Lausanne). 2020 May 22;11:329. doi: 10.3389/fendo.2020.00329. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32528416 Free PMC article. Review.
-
A new side-effect of sufentanil: increased monocyte-endothelial adhesion.BMC Anesthesiol. 2021 Nov 3;21(1):267. doi: 10.1186/s12871-021-01487-3. BMC Anesthesiol. 2021. PMID: 34732147 Free PMC article.
References
-
- Burnstock G. (2008). Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol. Rep. 60 12–20. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

