Orf Virus Encoded Protein ORFV119 Induces Cell Apoptosis Through the Extrinsic and Intrinsic Pathways
- PMID: 29896166
- PMCID: PMC5986898
- DOI: 10.3389/fmicb.2018.01056
Orf Virus Encoded Protein ORFV119 Induces Cell Apoptosis Through the Extrinsic and Intrinsic Pathways
Abstract
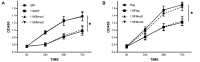
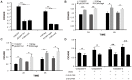
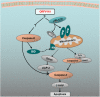
Apoptosis, a significant form of cell death, has a leading role in the host cell defense against virus infection. Viruses have evolved a series of strategies that block apoptosis during the early stage of viral infection to enhance viral replication, and induce apoptosis in the late stages to facilitate viral particle release from the cells. Here we show that orf virus (ORFV), the causative agent of orf, encodes an apoptosis-inducing protein ORFV119. ORFV119 targets the mitochondria in host cells, inhibits cell proliferation, and induces cell apoptosis. Protein array data indicated that ORFV119 could induce apoptosis via up-regulation of Smac, Bak, and Bax and down-regulation of anti-apoptotic proteins Bcl-2 and cIAP-2. Activation of caspase-9 and caspase-3, and consequent PARP cleavage, ultimately lead to apoptosis. ORFV119 could also directly activate caspase-8 and induce Bid, involved in the extrinsic pathway, to achieve cell death. Furthermore, sequence analysis and experiments with mutants of ORFV119 introduced revealed that ORFV119 contains a key N-terminal domain that is necessary and sufficient to direct the protein to the mitochondria. Together, we report, for the first time, the identification of the novel apoptosis-inducing protein ORFV119 encoded by a parapoxvirus. This provides an important reference for the study of pathogenesis, identification of immunomodulation mechanisms of ORFV, and may lead to new strategies for orf disease control.
Keywords: ORFV119; apoptosis; orf virus; parapoxvirus; protein array.
Figures






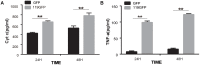
Similar articles
-
A parapoxviral virion protein targets the retinoblastoma protein to inhibit NF-κB signaling.PLoS Pathog. 2017 Dec 15;13(12):e1006779. doi: 10.1371/journal.ppat.1006779. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29244863 Free PMC article.
-
A novel Bcl-2-like inhibitor of apoptosis is encoded by the parapoxvirus ORF virus.J Virol. 2007 Jul;81(13):7178-88. doi: 10.1128/JVI.00404-07. Epub 2007 May 2. J Virol. 2007. PMID: 17475653 Free PMC article.
-
Induction of Apoptosis by the Nonstructural Protein 4 and 10 of Porcine Reproductive and Respiratory Syndrome Virus.PLoS One. 2016 Jun 16;11(6):e0156518. doi: 10.1371/journal.pone.0156518. eCollection 2016. PLoS One. 2016. PMID: 27310256 Free PMC article.
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
-
Apoptosis regulators.Rev Clin Exp Hematol. 2003 Jun;7(2):117-38. Rev Clin Exp Hematol. 2003. PMID: 14763159 Review.
Cited by
-
Human Infection with Orf Virus and Description of Its Whole Genome, France, 2017.Emerg Infect Dis. 2019 Dec;25(12):2197-2204. doi: 10.3201/eid2512.181513. Emerg Infect Dis. 2019. PMID: 31742503 Free PMC article.
-
Construction and characterization of a contagious ecthyma virus double-gene deletion strain and evaluation of its potential as a live-attenuated vaccine in goat.Front Immunol. 2022 Sep 2;13:961287. doi: 10.3389/fimmu.2022.961287. eCollection 2022. Front Immunol. 2022. PMID: 36119021 Free PMC article.
-
Pathological, immunological and molecular epidemiological analysis of lumpy skin disease virus in Indian cattle during a high-mortality epidemic.Vet Q. 2024 Dec;44(1):1-22. doi: 10.1080/01652176.2024.2398211. Epub 2024 Sep 5. Vet Q. 2024. PMID: 39233648 Free PMC article.
-
Orf Virus ORF120 Protein Positively Regulates the NF-κB Pathway by Interacting with G3BP1.J Virol. 2021 Sep 9;95(19):e0015321. doi: 10.1128/JVI.00153-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34287041 Free PMC article.
-
Genetic Variability of 3'-Proximal Region of Genomes of Orf Viruses Isolated From Sheep and Wild Japanese Serows (Capricornis crispus) in Japan.Front Vet Sci. 2020 Apr 24;7:188. doi: 10.3389/fvets.2020.00188. eCollection 2020. Front Vet Sci. 2020. PMID: 32391386 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

