Beneficial effects of dexmedetomidine on early postoperative cognitive dysfunction in pediatric patients with tonsillectomy
- PMID: 29896269
- PMCID: PMC5995088
- DOI: 10.3892/etm.2018.6180
Beneficial effects of dexmedetomidine on early postoperative cognitive dysfunction in pediatric patients with tonsillectomy
Abstract
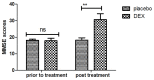
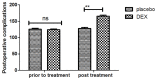
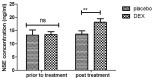
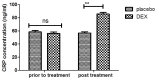
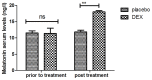
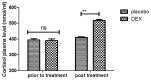
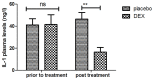
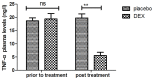
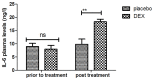
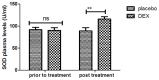
According to clinical investigations, early postoperative cognitive dysfunction is the most common adverse event in pediatric patients after tonsillectomy. A previous study has indicated that dexmedetomidine (DEX) is an efficient drug for the treatment of postoperative cognitive dysfunction. However, the efficacy of DEX in alleviating early postoperative cognitive dysfunction in pediatric patients following tonsillectomy has remained elusive, which was therefore assessed in the present study. A total of 186 children presenting with cognitive dysfunction subsequent to tonsillectomy were recruited to analyze the efficacy of DEX. Patients were randomly divided into two groups and received intravenous treatment with DEX (n=112) or placebo (n=74). Duration of treatment, dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) of DEX were evaluated in a preliminary experiment. The improvement of postoperative cognitive function in children with tonsillectomy was analyzed with a Mini-Mental State Examination (MMSE) following treatment with DEX. A 40-item quality of life (MONEX-40) questionnaire was used to assess the efficacy of DEX. The plasma levels of interleukin (IL)-6, IL-1, tumor necrosis factor (TNF)-α, superoxide dismutase (SOD), neuron-specific enolase (NSE), C-reactive protein (CRP), cortisol and melatonin were also analyzed. The preliminary experiment determined that the DLT was 10 mg/kg and the MTD was 15 mg/kg. In the major clinical trial, it was revealed that MMSE scores in the DEX treatment group were markedly improved, indicating that DEX had a beneficial effect in pediatric patients with early postoperative cognitive dysfunction after tonsillectomy. In addition, IL-1and TNF-α were downregulated, while IL-6 and SOD were upregulated in patients with cognitive dysfunction after treatment with DEX compared with those in the placebo group. Furthermore, DEX treatment markedly decreased the serum levels of CRP, NSE cortisol and melatonin, which are associated with the occurrence of postoperative cognitive dysfunction in pediatric patients following tonsillectomy. In conclusion, intravenous administration of DEX at a dose of 10 mg/kg improves postoperative cognitive function in pediatric patients with tonsillectomy by decreasing the serum levels of inflammatory factors and stress-associated signaling molecules. Trial registration no. QLSDHOS0200810102C (Qilu Hospital of Shandong University, Jinan, China).
Keywords: dexmedetomidine; inflammation; postoperative cognitive dysfunction; tonsillectomy.
Figures
Similar articles
-
Effects of Dexmedetomidine Anesthesia on Early Postoperative Cognitive Dysfunction in Elderly Patients.ACS Chem Neurosci. 2022 Aug 3;13(15):2309-2314. doi: 10.1021/acschemneuro.2c00173. Epub 2022 Jul 21. ACS Chem Neurosci. 2022. PMID: 35864562 Clinical Trial.
-
Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri-operative inflammation in elderly patients undergoing laparoscopic cholecystectomy.Exp Ther Med. 2015 Nov;10(5):1635-1642. doi: 10.3892/etm.2015.2726. Epub 2015 Sep 4. Exp Ther Med. 2015. PMID: 26640530 Free PMC article.
-
[Effects of dexmedetomidine doses on postoperative cognitive dysfunction and serum β- amyloid and cytokine levels in elderly patients after spine surgery: a randomized controlled trial].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Apr 20;41(4):600-606. doi: 10.12122/j.issn.1673-4254.2021.04.18. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 33963722 Free PMC article. Clinical Trial. Chinese.
-
Effects of dexmedetomidine on early postoperative cognitive function and postoperative inflammatory response: a systematic review and network meta-analysis.Front Neurol. 2024 Aug 12;15:1422049. doi: 10.3389/fneur.2024.1422049. eCollection 2024. Front Neurol. 2024. PMID: 39188709 Free PMC article.
-
Dexmedetomidine May Reduce IL-6 Level and the Risk of Postoperative Cognitive Dysfunction in Patients After Surgery: A Meta-Analysis.Dose Response. 2020 Feb 5;18(1):1559325820902345. doi: 10.1177/1559325820902345. eCollection 2020 Jan-Mar. Dose Response. 2020. PMID: 32076394 Free PMC article. Review.
Cited by
-
The role of anesthesia in peri‑operative neurocognitive disorders: Molecular mechanisms and preventive strategies.Fundam Res. 2023 Feb 24;4(4):797-805. doi: 10.1016/j.fmre.2023.02.007. eCollection 2024 Jul. Fundam Res. 2023. PMID: 39161414 Free PMC article. Review.
-
Dexmedetomidine as an Adjuvant to Nerve Block for Cancer Surgery: A Systematic Review and Meta-Analysis.J Clin Med. 2024 May 28;13(11):3166. doi: 10.3390/jcm13113166. J Clin Med. 2024. PMID: 38892876 Free PMC article. Review.
-
Serum Inflammatory Factors and Oxidative Stress Factors Are Associated With Increased Risk of Frailty and Cognitive Frailty in Patients With Cerebral Small Vessel Disease.Front Neurol. 2022 Jan 6;12:786277. doi: 10.3389/fneur.2021.786277. eCollection 2021. Front Neurol. 2022. PMID: 35069415 Free PMC article.
-
Regulatory role of microRNA-320 during off-pump coronary artery bypass grafting with dexmedetomidine adjunct anesthesia.Exp Ther Med. 2021 Nov;22(5):1201. doi: 10.3892/etm.2021.10635. Epub 2021 Aug 23. Exp Ther Med. 2021. PMID: 34584546 Free PMC article.
-
Protective effect of dexmedetomidine infusion combined with epidural blockade on postoperative complications after surgery: A prospective randomized controlled clinical trial.J Int Med Res. 2020 Jun;48(6):300060520930168. doi: 10.1177/0300060520930168. J Int Med Res. 2020. PMID: 32579483 Free PMC article. Clinical Trial.
References
-
- Parker D, Howe L, Unsworth V, Hilliam R. A randomised controlled trial to compare postoperative pain in children undergoing tonsillectomy using cold steel dissection with bipolar haemostasis versus coblation technique. Clin Otolaryngol. 2009;34:225–231. doi: 10.1111/j.1749-4486.2009.01932.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
