Multimodality reporter gene imaging: Construction strategies and application
- PMID: 29896296
- PMCID: PMC5996353
- DOI: 10.7150/thno.24108
Multimodality reporter gene imaging: Construction strategies and application
Abstract
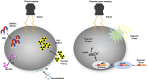
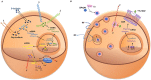
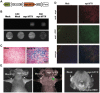
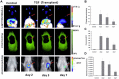
Molecular imaging has played an important role in the noninvasive exploration of multiple biological processes. Reporter gene imaging is a key part of molecular imaging. By combining with a reporter probe, a reporter protein can induce the accumulation of specific signals that are detectable by an imaging device to provide indirect information of reporter gene expression in living subjects. There are many types of reporter genes and each corresponding imaging technique has its own advantages and drawbacks. Fused reporter genes or single reporter genes with products detectable by multiple imaging modalities can compensate for the disadvantages and potentiate the advantages of each modality. Reporter gene multimodality imaging could be applied to trace implanted cells, monitor gene therapy, assess endogenous molecular events, screen drugs, etc. Although several types of multimodality imaging apparatus and multimodality reporter genes are available, more sophisticated detectors and multimodality reporter gene systems are needed.
Keywords: cell tracing; drug screening; gene directed therapy; molecular imaging; multimodality imaging; reporter gene.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Rational design of a triple reporter gene for multimodality molecular imaging.Biomed Res Int. 2014;2014:605358. doi: 10.1155/2014/605358. Epub 2014 Apr 7. Biomed Res Int. 2014. PMID: 24809057 Free PMC article.
-
Molecular-genetic imaging based on reporter gene expression.J Nucl Med. 2008 Jun;49 Suppl 2:164S-79S. doi: 10.2967/jnumed.107.045955. J Nucl Med. 2008. PMID: 18523072 Review.
-
Reporter gene imaging.AJR Am J Roentgenol. 2013 Aug;201(2):W206-14. doi: 10.2214/AJR.13.10555. AJR Am J Roentgenol. 2013. PMID: 23883235 Review.
-
MRI Reporter Genes for Noninvasive Molecular Imaging.Molecules. 2016 May 18;21(5):580. doi: 10.3390/molecules21050580. Molecules. 2016. PMID: 27213309 Free PMC article. Review.
-
Noninvasive molecular imaging using reporter genes.J Nucl Med. 2013 Feb;54(2):167-72. doi: 10.2967/jnumed.111.099788. Epub 2013 Jan 14. J Nucl Med. 2013. PMID: 23318292 Review.
Cited by
-
Reporter Genes for Brain Imaging Using MRI, SPECT and PET.Int J Mol Sci. 2022 Jul 30;23(15):8443. doi: 10.3390/ijms23158443. Int J Mol Sci. 2022. PMID: 35955578 Free PMC article. Review.
-
PET imaging of AAV9 and AAVBR1 trafficking in normal mice.Sci Rep. 2025 Feb 4;15(1):4257. doi: 10.1038/s41598-025-86815-0. Sci Rep. 2025. PMID: 39905154 Free PMC article.
-
Development of a Human Photoacoustic Imaging Reporter Gene Using the Clinical Dye Indocyanine Green.Radiol Imaging Cancer. 2019 Nov 29;1(2):e190035. doi: 10.1148/rycan.2019190035. eCollection 2019 Nov. Radiol Imaging Cancer. 2019. PMID: 33778683 Free PMC article.
-
A Novel Luciferase-Based Reporter Gene Technology for Simultaneous Optical and Radionuclide Imaging of Cells.Int J Mol Sci. 2024 Jul 27;25(15):8206. doi: 10.3390/ijms25158206. Int J Mol Sci. 2024. PMID: 39125775 Free PMC article.
-
Reporter gene systems for the identification and characterization of cancer stem cells.World J Stem Cells. 2021 Jul 26;13(7):861-876. doi: 10.4252/wjsc.v13.i7.861. World J Stem Cells. 2021. PMID: 34367481 Free PMC article. Review.
References
-
- Henriquez NV, van Overveld PG, Que I, Buijs JT, Bachelier R, Kaijzel EL. et al. Advances in optical imaging and novel model systems for cancer metastasis research. Clin Exp Metastasis. 2007;24:699–705. - PubMed
-
- Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–33. - PubMed
-
- Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. Jama. 2005;293:855–62. - PubMed
-
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–80. - PubMed
-
- Thakur M, Lentle BC. Report of a summit on molecular imaging. Radiology. 2005;236:753–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

