Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications
- PMID: 29896301
- PMCID: PMC5996358
- DOI: 10.7150/thno.23459
Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications
Abstract
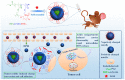
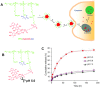
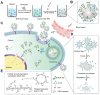
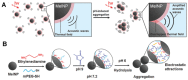
Nanotechnology-based antitumor drug delivery systems, known as nanocarriers, have demonstrated their efficacy in recent years. Typically, the size of the nanocarriers is around 100 nm. It is imperative to achieve an optimum size of these nanocarriers which must be designed uniquely for each type of delivery process. For pH-responsive nanocarriers with programmable size, changes in pH (~6.5 for tumor tissue, ~5.5 for endosomes, and ~5.0 for lysosomes) may serve as an endogenous stimulus improving the safety and therapeutic efficacy of antitumor drugs. This review focuses on current advanced pH-responsive nanocarriers with programmable size changes for anticancer drug delivery. In particular, pH-responsive mechanisms for nanocarrier retention at tumor sites, size reduction for penetrating into tumor parenchyma, escaping from endo/lysosomes, and swelling or disassembly for drug release will be highlighted. Additional trends and challenges of employing these nanocarriers in future clinical applications are also addressed.
Keywords: endogenous pH-responsive; nanocarriers; size change; targeted drug delivery; tumor therapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures










References
-
- Duan XP, Li YP. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small. 2013;9:1521–32. - PubMed
-
- King MR, Mohamed ZJ. Dual nanoparticle drug delivery: the future of anticancer therapies? Nanomedicine. 2017;12:95–8. - PubMed
-
- Du YF, Chen W, Zheng M, Meng FH, Zhong ZY. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials. 2012;33:7291–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

