Dual-functional protein for one-step production of a soluble and targeted fluorescent dye
- PMID: 29896306
- PMCID: PMC5996361
- DOI: 10.7150/thno.24613
Dual-functional protein for one-step production of a soluble and targeted fluorescent dye
Abstract
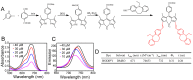
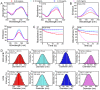
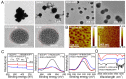
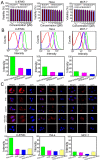
Low water solubility and poor selectivity are two fundamental limitations that compromise applications of near-infrared (NIR) fluorescent probes. Methods: Here, a simple strategy that can resolve these problems simultaneously was developed by using a novel hybrid protein named RGD-HFBI that is produced by fusion of hydrophobin HFBI and arginine-glycine-aspartic acid (RGD) peptide. This unique hybrid protein inherits self-assembly and targeting functions from HFBI and RGD peptide respectively. Results: Boron-dipyrromethene (BODIPY) used as a model NIR dye can be efficiently dispersed in the RGD-HFBI solution by simple mixing and sonication for 30 min. The data shows that self-assembled RGD-HFBI forms a protein nanocage by using the BODIPY as the assembly template. Cell uptake assay proves that RGD-HFBI/BODIPY can efficiently stain αvβ3 integrin-positive cancer cells. Finally, in vivo affinity tests fully demonstrate that the soluble RGD-HFBI/BODIPY complex selectively targets and labels tumor sites of tumor-bearing mice due to the high selectivity of the RGD peptide. Conclusion: Our one-step strategy using dual-functional RGD-HFBI opens a novel route to generate soluble and targeted NIR fluorescent dyes in a very simple and efficient way and may be developed as a general strategy to broaden their applications.
Keywords: NIR fluorescent probes; RGD peptide; protein nanocage; self-assembly.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Highly water-soluble, near-infrared emissive BODIPY polymeric dye bearing RGD peptide residues for cancer imaging.Anal Chim Acta. 2013 Jan 3;758:138-44. doi: 10.1016/j.aca.2012.10.026. Epub 2012 Nov 1. Anal Chim Acta. 2013. PMID: 23245906 Free PMC article.
-
Synthesis and evaluation of a novel BODIPY fluorescent probe targeting integrin αvβ3 for cancer diagnosis.Eur J Med Chem. 2025 Jan 15;282:117056. doi: 10.1016/j.ejmech.2024.117056. Epub 2024 Nov 13. Eur J Med Chem. 2025. PMID: 39549324
-
Self-assembled hydrophobin for producing water-soluble and membrane permeable fluorescent dye.Sci Rep. 2016 Mar 15;6:23061. doi: 10.1038/srep23061. Sci Rep. 2016. PMID: 26976627 Free PMC article.
-
Integrin (αvβ3) Targeted RGD Peptide Based Probe for Cancer Optical Imaging.Curr Protein Pept Sci. 2016;17(6):570-81. doi: 10.2174/1389203717666160101124015. Curr Protein Pept Sci. 2016. PMID: 26721402 Review.
-
Tailoring nanoparticles based on boron dipyrromethene for cancer imaging and therapy.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Jul;12(4):e1627. doi: 10.1002/wnan.1627. Epub 2020 Mar 12. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020. PMID: 32164043 Review.
Cited by
-
Peptide-Directed Synthesis of Aggregation-Induced Emission Enhancement-Active Gold Nanoclusters for Single- and Two-Photon Imaging of Lysosome and Expressed αvβ3 Integrin Receptors.Anal Chem. 2024 Jun 4;96(22):9007-9015. doi: 10.1021/acs.analchem.4c00321. Epub 2024 May 23. Anal Chem. 2024. PMID: 38778775 Free PMC article.
-
Targeted and pH-facilitated theranostic of orthotopic gastric cancer via phase-transformation doxorubicin-encapsulated nanoparticles enhanced by low-intensity focused ultrasound (LIFU) with reduced side effect.Int J Nanomedicine. 2019 Sep 18;14:7627-7642. doi: 10.2147/IJN.S212888. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31571868 Free PMC article.
-
Advances in intracellular delivery through supramolecular self-assembly of oligonucleotides and peptides.Theranostics. 2019 May 18;9(11):3191-3212. doi: 10.7150/thno.33921. eCollection 2019. Theranostics. 2019. PMID: 31244949 Free PMC article. Review.
-
SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury.Aging (Albany NY). 2020 Jul 28;12(16):16224-16237. doi: 10.18632/aging.103644. Epub 2020 Jul 28. Aging (Albany NY). 2020. PMID: 32721927 Free PMC article.
-
Biodegradation of highly crystallized poly(ethylene terephthalate) through cell surface codisplay of bacterial PETase and hydrophobin.Nat Commun. 2022 Nov 21;13(1):7138. doi: 10.1038/s41467-022-34908-z. Nat Commun. 2022. PMID: 36414665 Free PMC article.
References
-
- Guo Z, Park S, Yoon J, Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev. 2014;43:16–29. - PubMed
-
- Sato R, Kozuka J, Ueda M, Mishima R, Kumagai Y, Yoshimura A. et al. Intracellular protein-labeling probes for multicolor single-molecule imaging of immune receptor-adaptor molecular dynamics. J Am Chem Soc. 2017;139:17397–404. - PubMed
-
- Evans CL. New near-infrared dyes light up deep tissue imaging. Sci Transl Med. 2017;9:eaam6065. - PubMed
-
- Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

