A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction
- PMID: 29896309
- PMCID: PMC5996367
- DOI: 10.7150/thno.22080
A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction
Erratum in
-
Erratum: A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction.Theranostics. 2018 Jul 27;8(15):4152-4154. doi: 10.7150/thno.28368. eCollection 2018. Theranostics. 2018. PMID: 30128043 Free PMC article.
Abstract
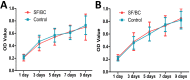
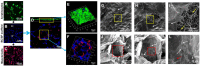
Rationale: In urethral tissue engineering, the currently available reconstructive procedures are insufficient due to a lack of appropriate scaffolds that would support the needs of various cell types. To address this problem, we developed a bilayer scaffold comprising a microporous network of silk fibroin (SF) and a nanoporous bacterial cellulose (BC) scaffold and evaluated its feasibility and potential for long-segment urethral regeneration in a dog model. Methods: The freeze-drying and self-assembling method was used to fabricate the bilayer scaffold by stationary cultivation G. xylinus using SF scaffold as a template. The surface morphology, porosity and mechanical properties of all prepared SF-BC scaffolds were characterized using Scanning electron microscopy (SEM), microcomputed tomography and universal testing machine. To further investigate the suitability of the bilayer scaffolds for tissue engineering applications, biocompatibility was assessed using an MTT assay. The cell distribution, viability and morphology were evaluated by seeding epithelial cells and muscle cells on the scaffolds, using the 3D laser scanning confocal microscopy, and SEM. The effects of urethral reconstruction with SF-BC bilayer scaffold was evaluated in dog urethral defect models. Results: Scanning electron microscopy revealed that SF-BC scaffold had a clear bilayer structure. The SF-BC bilayer scaffold is highly porous with a porosity of 85%. The average pore diameter of the porous layer in the bilayer SF-BC composites was 210.2±117.8 μm. Cultures established with lingual keratinocytes and lingual muscle cells confirmed the suitability of the SF-BC structures to support cell adhesion and proliferation. In addition, SEM demonstrated the ability of cells to attach to scaffold surfaces and the biocompatibility of the matrices with cells. At 3 months after implantation, urethra reconstructed with the SF-BC scaffold seeded with keratinocytes and muscle cells displayed superior structure compared to those with only SF-BC scaffold. Principal Conclusion: These results demonstrate that the bilayer SF-BC scaffold may be a promising biomaterial with good biocompatibility for urethral regeneration and could be used for numerous other types of hollow-organ tissue engineering grafts, including vascular, bladder, ureteral, bowel, and intestinal.
Keywords: bacterial cellulose; bilayer scaffold; lingual keratinocytes; muscle cells; silk fibroin; urethral reconstruction.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model.Biomed Mater. 2015 Sep 11;10(5):055005. doi: 10.1088/1748-6041/10/5/055005. Biomed Mater. 2015. PMID: 26358641
-
Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model.J Surg Res. 2014 May 1;188(1):1-7. doi: 10.1016/j.jss.2013.11.1102. Epub 2013 Dec 6. J Surg Res. 2014. PMID: 24411303
-
Silk fibroin/collagen and silk fibroin/chitosan blended three-dimensional scaffolds for tissue engineering.Eur J Orthop Surg Traumatol. 2015 Feb;25(2):243-9. doi: 10.1007/s00590-014-1515-z. Epub 2014 Aug 14. Eur J Orthop Surg Traumatol. 2015. PMID: 25118870
-
Silk Fibroin-Based Biomaterials for Tissue Engineering Applications.Molecules. 2022 Apr 25;27(9):2757. doi: 10.3390/molecules27092757. Molecules. 2022. PMID: 35566110 Free PMC article. Review.
-
Silk Fibroin Scaffolds for Urologic Tissue Engineering.Curr Urol Rep. 2016 Feb;17(2):16. doi: 10.1007/s11934-015-0567-x. Curr Urol Rep. 2016. PMID: 26801192 Free PMC article. Review.
Cited by
-
Prospects and Challenges of Electrospun Cell and Drug Delivery Vehicles to Correct Urethral Stricture.Int J Mol Sci. 2022 Sep 10;23(18):10519. doi: 10.3390/ijms231810519. Int J Mol Sci. 2022. PMID: 36142432 Free PMC article. Review.
-
An overview to nanocellulose clinical application: Biocompatibility and opportunities in disease treatment.Regen Ther. 2023 Nov 16;24:630-641. doi: 10.1016/j.reth.2023.10.006. eCollection 2023 Dec. Regen Ther. 2023. PMID: 38034858 Free PMC article. Review.
-
Generation of Cost-Effective Paper-Based Tissue Models through Matrix-Assisted Sacrificial 3D Printing.Nano Lett. 2019 Jun 12;19(6):3603-3611. doi: 10.1021/acs.nanolett.9b00583. Epub 2019 May 7. Nano Lett. 2019. PMID: 31010289 Free PMC article.
-
Bilayer Scaffolds for Interface Tissue Engineering and Regenerative Medicine: A Systematic Reviews.Adv Exp Med Biol. 2021;1347:83-113. doi: 10.1007/5584_2021_637. Adv Exp Med Biol. 2021. PMID: 33931833
-
Construction of Tissue-Engineered Bladder Scaffolds with Composite Biomaterials.Polymers (Basel). 2022 Jun 29;14(13):2654. doi: 10.3390/polym14132654. Polymers (Basel). 2022. PMID: 35808700 Free PMC article.
References
-
- Andrich DE, Mundy AR. Substitution urethroplasty with buccal mucosal-free grafts. J Urol. 2001;165:1131–4. - PubMed
-
- Bhargava S, Chapple CR. Buccal mucosal urethroplasty: is it the new gold standard? BJU Int. 2004;93:1191–3. - PubMed
-
- Xu YM, Qiao Y, Sa YL, Zhang J, Fu Q, Song LJ. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol. 2009;182:1040–3. - PubMed
-
- Jang TL, Erickson B, Medendorp A, Gonzalez CM. Comparison of donor site intraoral morbidity after mucosal graft harvesting for urethral reconstruction. Urology. 2005;66:716–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

