Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes
- PMID: 29896310
- PMCID: PMC5996368
- DOI: 10.7150/thno.22164
Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes
Abstract
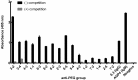
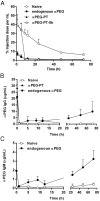
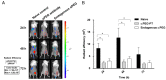
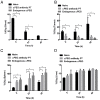
Rationale: Increasing frequency of human exposure to PEG-related products means that healthy people are likely to have pre-existing anti-PEG antibodies (pre-αPEG Ab). However, the influence of pre-αPEG Abs on the pharmacokinetics (PK) and therapeutic efficacy of LipoDox is unknown. Methods: We generated two pre-αPEG Ab mouse models. First, naïve mice were immunized with PEGylated protein to generate an endogenous αPEG Ab titer (endo αPEG). Second, monoclonal αPEG Abs were passively transferred (αPEG-PT) into naïve mice to establish a αPEG titer. The naïve, endo αPEG and αPEG-PT mice were intravenously injected with 111in-labeled LipoDox to evaluate its PK. Tumor-bearing naïve, endo αPEG and αPEG-PT mice were intravenously injected with 111in-labeled LipoDox to evaluate its biodistribution. The therapeutic efficacy of LipoDox was estimated in the tumor-bearing mice. Results: The areas under the curve (AUC)last of LipoDox in endo αPEG and αPEG-PT mice were 11.5- and 15.6- fold less, respectively, than that of the naïve group. The biodistribution results suggested that pre-αPEG Ab can significantly reduce tumor accumulation and accelerate blood clearance of 111In-labeled LipoDox from the spleen. The tumor volumes of the tumor-bearing endo αPEG and αPEG-PT mice after treatment with LipoDox were significantly increased as compared with that of the tumor-bearing naïve mice. Conclusions: Pre-αPEG Abs were found to dramatically alter the PK and reduce the tumor accumulation and therapeutic efficacy of LipoDox. Pre-αPEG may have potential as a marker to aid development of personalized therapy using LipoDox and achieve optimal therapeutic efficacy.
Keywords: ELISA; LipoDox; PEG; Polyethylene glycol; anti-PEG antibodies; liposome.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Clinical Relevance of Pre-Existing and Treatment-Induced Anti-Poly(Ethylene Glycol) Antibodies.Regen Eng Transl Med. 2022;8(1):32-42. doi: 10.1007/s40883-021-00198-y. Epub 2021 Mar 25. Regen Eng Transl Med. 2022. PMID: 33786367 Free PMC article. Review.
-
Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models.J Drug Target. 2002 Nov;10(7):539-48. doi: 10.1080/1061186021000072447. J Drug Target. 2002. PMID: 12683721
-
Lipodox® (generic doxorubicin hydrochloride liposome injection): in vivo efficacy and bioequivalence versus Caelyx® (doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models.BMC Cancer. 2017 Jun 6;17(1):405. doi: 10.1186/s12885-017-3377-3. BMC Cancer. 2017. PMID: 28587612 Free PMC article.
-
Comparative plasma and tissue distribution of Sun Pharma's generic doxorubicin HCl liposome injection versus Caelyx® (doxorubicin HCl liposome injection) in syngeneic fibrosarcoma-bearing BALB/c mice and Sprague-Dawley rats.Cancer Chemother Pharmacol. 2017 May;79(5):899-913. doi: 10.1007/s00280-017-3278-9. Epub 2017 Mar 27. Cancer Chemother Pharmacol. 2017. PMID: 28349166 Free PMC article.
-
Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours.Drugs. 1997;54 Suppl 4:15-21. doi: 10.2165/00003495-199700544-00005. Drugs. 1997. PMID: 9361957 Review.
Cited by
-
Peptide functionalized liposomes for receptor targeted cancer therapy.APL Bioeng. 2021 Jan 25;5(1):011501. doi: 10.1063/5.0029860. eCollection 2021 Mar. APL Bioeng. 2021. PMID: 33532673 Free PMC article. Review.
-
Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology.ACS Nano. 2020 Aug 25;14(8):9221-9227. doi: 10.1021/acsnano.0c04753. Epub 2020 Jul 24. ACS Nano. 2020. PMID: 32706238 Free PMC article.
-
Enhancement of tumor tropism of mPEGylated nanoparticles by anti-mPEG bispecific antibody for ovarian cancer therapy.Sci Rep. 2021 Apr 7;11(1):7598. doi: 10.1038/s41598-021-87271-2. Sci Rep. 2021. PMID: 33828191 Free PMC article.
-
Clinical Relevance of Pre-Existing and Treatment-Induced Anti-Poly(Ethylene Glycol) Antibodies.Regen Eng Transl Med. 2022;8(1):32-42. doi: 10.1007/s40883-021-00198-y. Epub 2021 Mar 25. Regen Eng Transl Med. 2022. PMID: 33786367 Free PMC article. Review.
-
Flow cytometry analysis of anti-polyethylene glycol antibodies in human plasma.Toxicol Rep. 2020 Dec 26;8:148-154. doi: 10.1016/j.toxrep.2020.12.022. eCollection 2021. Toxicol Rep. 2020. PMID: 33437656 Free PMC article.
References
-
- Koning GA, Morselt HW, Kamps JA, Scherphof GL. Uptake and intracellular processing of PEG-liposomes and PEG-immunoliposomes by kupffer cells in vitro 1 *. J Liposome Res. 2001;11:195–209. - PubMed
-
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–7. - PubMed
-
- Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat. 2016;29:90–106. - PubMed
-
- Poizot-Martin I, Giovannini M, Rosello R, Viallat JR, Sauniere JF, Dalmas AM. et al. Liposomal-doxorubicin in human immunodeficiency virus-associated Kaposi's sarcoma. J Clin Oncol. 1994;12:645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

